- Nonactin
-
Nonactin 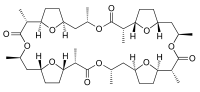
Identifiers CAS number 6833-84-7 
PubChem 72519 ChEMBL CHEMBL415914 
Jmol-3D images Image 1 - CC1CC2CCC(O2)C(C(=O)OC(CC3CCC(O3)C(C(=O)OC(CC4CCC(O4)C(C(=O)OC(CC5CCC(O5)C(C(=O)O1)C)C)C)C)C)C)C
Properties Molecular formula C40H64O12 Molar mass 736.92896 g/mol Melting point 146 °C, 419 K, 295 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nonactin is a member of a family of naturally occurring cyclic ionophores known as the macrotetrolide antibiotics. The other members of nactins homologous family are monactin, dinactin, trinactin and tetranactin which are all neutral ionophoric substances and higher homologs of nonactin. Collectively, this class is known as the nactins. Nonactin is soluble in methanol, dichloromethane, ethyl acetate and DMSO, but insoluble in water.
Contents
Sources
Nonactin is commercially available; as of 2006, there are three bacterial species that produce nonactin: Streptomyces tsukubaensis, Streptomyces griseus, Streptomyces chrysomallus and Streptomyces werraensis. Total syntheses have been reported.[1][2]
Structure and properties
Nonactin was isolated by Corbaz et al. in 1955 from bacterial strains.[3] It is composed of four tetrahydrofuran rings and four esters linked by saturated aliphatic chain sections. Nonactin has a 48-member ring, built from 40 carbon and 12 (8 on the ring, 4 as ketones) oxygen atoms. Liquid chromatography-mass spectrometry (LC-MS) offers a modern approach to obtain more detailed process control data than the spectrophotometric and chromatographic measurements used in the past.[4]
Reactions
Nonactin is known for its ability to form complexes with alkali cations, most notably potassium and sodium. In general, nonactin (and other members of the nactin family) exhibits binding preferences for some ions over others. This ion selectivity is seen in other macrocyclic ligands such as the cyclic ionophore valinomycin, which is also an antibiotic, and crown ethers. Although nonactin (and in fact, all nactins) exhibits an especially high cation selectivity for potassium ions over sodium ions or rubidium ions, it exhibits the highest selectivity for ammonium ions and thallium ions.
Due to this property, Nonactin is also called "Ammonium ionophore"[5][6]
During complexation, the nonactin backbone convolutes into a pattern resembling the seam of a tennis ball. In the potassium-nonactin complex, the potassium ion is entirely surrounded by four carbonyl oxygen atoms and the four oxygen atoms of the tetrahydrofuran ring. These eight oxygen atoms surrounding the ion are nearly equidistant from it and adopt a nearly cubic coordination sphere around the ion. In this complex, all polar carbonyl groups point inwards and nonpolar moieties point outwards, thus building up a hydrophobic exterior for the complex and making it soluble in lipid membranes. This is how nonactin is able to transport potassium ions across lipid membranes.
Biological effects
Nonactin has been reported to specifically inhibit the processing of cytoplasmic precursor proteins destined for the mitochondria. It is able to uncouple the oxidative phosphorylation of mitochondria of rat liver in a low concentration, and can also carry cations across biological and artificial membranes.[citation needed]
A nactins mixture, purposely enriched in tetranactin and poor in nonactin, known as Polynactin(c), was used as a pesticide, but since 2004 is not used any more, presumably because its residuals appeared in food.[7]
Applications
There is no known medical use of nactins. Ultrapure nonactin, practically free of other nactins, is used for ammonium specific electrodes.
References
- ^ Ian Fleming and Sunil K. Ghosh (1994). "A total synthesis of nonactin". Journal of the Chemical Society Chemical Communications (19): 2287. doi:10.1039/C39940002287.
- ^ Ju Y.L.; Byeang H.K. (1996). "Total synthesis of nonactin". Tetrahedron 52 (2): 571. doi:10.1016/0040-4020(95)00913-2.
- ^ R. Corbaz, L. Ettlinger, E. Gäumann, W. Keller-Schierlein, F. Kradolfer, L. Neipp, V. Prelog, H. Zähner (1955). "Stoffwechselprodukte von Actinomyceten. 3. Mitteilung. Nonactin". Helvetica Chimica Acta 38 (6): 1445–1448. doi:10.1002/hlca.19550380617.
- ^ Jani P., Emmert J., Wohlgemuth R. (2008). "Process analysis of macrotetrolide biosynthesis during fermentation by means of direct infusion LC-MS". Biotechn. J. 3 (2): 202–208. doi:10.1002/biot.200700174. PMID 18064609.
- ^ Nonactin product page from Fermentek
- ^ Nonactin Bulletin
- ^ Notification, the World Trade Organization's revocation of Polynactin agricultural usage, July 20, 2004.
Further reading
- Vishwanath, C. K.; Shamala, N.; Easwaran, K. R. K.; Vijayan, M. (1983). "Structure of nonactin–calcium perchlorate, C40H64O12.Ca(ClO4)2, and a comparative study of metal–nonactin complexes". Acta Crystallographica C 39 (12): 1640. doi:10.1107/S0108270183009580.
- Woo AJ, Strohl WR, Priestley ND (July 1999). "Nonactin biosynthesis: the product of nonS catalyzes the formation of the furan ring of nonactic acid". Antimicrob. Agents Chemother. 43 (7): 1662–8. PMC 89340. PMID 10390219. http://aac.asm.org/cgi/pmidlookup?view=long&pmid=10390219.
Categories:- Biomolecules
- Ionophores
Wikimedia Foundation. 2010.
