- Titanium(II) chloride
-
Titanium(II) chloride 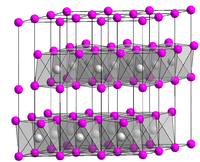
Identifiers CAS number 10049-06-6 PubChem 66228 ChemSpider 8466246 
Jmol-3D images Image 1 - [Ti+2].[Cl-].[Cl-]
Properties Molecular formula Cl2Ti Molar mass 118.77 g mol−1 Appearance black hexagonal crystals Density 3.13 g/cm3 Melting point 1035°C
Boiling point 1500°C[1]
Hazards MSDS External MSDS EU classification  Xn
Xn  C
C chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Titanium(II) chloride is the chemical compound with the formula TiCl2. The black solid has been studied only moderately, probably because of its high reactivity.[2] Ti(II) is a strong reducing agent: it has a high affinity for oxygen and reacts irreversibly with water to produce H2. The usual preparation is the thermal disproportionation of TiCl3 at 500 °C. The equilibrium is "driven" by the loss of volatile TiCl4:
-
- 2 TiCl3 → TiCl2 + TiCl4
The method is similar to that for the conversion of VCl3 into VCl2 and VCl4.
TiCl2 crystallizes as the layered CdI2 structure. Thus, the Ti(II) centers are octahedrally coordinated to six chloride ligands.[3][4]
Derivatives
Molecular complexes are known such as TiCl2(chel)2, where chel is DMPE ((CH3)2PCH2CH2P(CH3)2 and TMEDA ((CH3)2NCH2CH2N(CH3)2).[5] Such species are prepared by reduction of related Ti(III) and Ti(IV) complexes.
Unusual electronic effects have been observed in these species: TiCl2[(CH3)2PCH2CH2P(CH3)2]2 is paramagnetic with a triplet ground state, but Ti(CH3)2[(CH3)2PCH2CH2P(CH3)2]2 is diamagnetic.[6]
A solid-state derivative of TiCl2 is Na2TiCI4, which has been prepared by the reaction of Ti metal with TiCl3 in a NaCl flux.[7] This species adopts a linear chain structure wherein again the Ti(II) centers are octahedral with terminal, axial halides.[8]
References
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 491. ISBN 0-8493-0594-2
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Gal'perin, E. L.; Sandler, R. A. (1962). "TiCI2". Kristallografiya 7: 217–19..
- ^ Baenziger, N. C.; Rundle, R. E. (1948). "TiCI2". Acta Cryst. 1 (5): 274–274. doi:10.1107/S0365110X48000740.
- ^ Girolami, G. S.; Wilkinson, G.; Galas, A. M. R.; Thornton-Pett, M.; Hursthouse, M. B. (1985). "Synthesis and properties of the divalent 1,2-bis(dimethylphosphino)ethane (dmpe) complexes MCl2(dmpe)2 and MMe2(dmpe)2 (M = Ti, V, Cr, Mn, or Fe). X-Ray crystal structures of MCl2(dmpe)2 (M = Ti, V, or Cr), MnBr2(dmpe)2, TiMe1.3Cl0.7(dmpe)2, and CrMe2(dmpe)2". J. Chem. Soc., Dalton Transactions: 1339–1348.
- ^ Jensen, J. A.; Wilson, S. R.; Schultz, A. J.; Girolami, G. S. (1987). "Divalent Titanium Chemistry. Synthesis, Reactivity, and X-ray and Neutron Diffraction Studies of Ti(BH4)2(dmpe)2 and Ti(CH3)2(dmpe)2". J. Am. Chem. Soc. 109 (26): 8094–5. doi:10.1021/ja00260a029.
- ^ Hinz, D. J.; Dedecke, T.; Urland, W.; Meyer, G.. "Synthese, Kristallstruktur und Magnetismus von Natriumtetrachlorotitanat(lI), Na2TiCI4". ZAAC 620: 801–804.
- ^ Jongen, L.; Gloger, T.; Beekhuizen, J. and Meyer, G. (2005). "Divalent titanium: The halides ATiX3 (A = K, Rb, Cs; X = Cl, Br, I)". ZAAC 631 (2–3): 582–586. doi:10.1002/zaac.200400464.
Titanium compounds Categories:- Metal halides
- Chlorides
- Titanium compounds
Wikimedia Foundation. 2010.
