- Vanadocene dichloride
-
Vanadocene dichloride 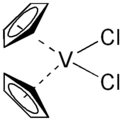
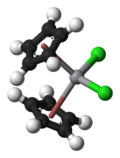 dichloro bis (η5-cyclopentadienyl) vanadiumOther namesDicyclopentadienyl vanadium dichloride
dichloro bis (η5-cyclopentadienyl) vanadiumOther namesDicyclopentadienyl vanadium dichlorideIdentifiers Abbreviations Cp2VCl2 CAS number 12083-48-6 RTECS number YW1580000 Properties Molecular formula C10H10Cl2V Molar mass 252.03 g/mol Appearance Green solid Density 1.7 g/ml Melting point Decomp.
Boiling point Decomp.
Solubility in water Soluble (Hydrolysis) Structure Crystal structure Monoclinic Coordination
geometryTetrahedral Hazards R-phrases R25 R36/37/38 R38 S-phrases S26 S28 S36/37/39 S45 Main hazards Irritant NFPA 704 Related compounds Related compounds Titanocene dichloride
Zirconocene dichloride
Hafnocene dichloride
Niobocene dichloride
Tanatalocene dichloride
Molybdenocene dichloride
Tungstenocene dichloride dichloride (verify) (what is:
dichloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Vanadocene dichloride, dichloro bis(η5-cyclopentadienyl)vanadium(IV) is (η5-C5H5)2VCl2 (commonly abbreviated as Cp2VCl2). It is a structural analoque of titanocene dichloride but with vanadium(IV) instead of titanium(IV). This compound has one unpaired electron, hence Cp2VCl2 is paramagnetic. Vanadocene dichloride is a suitable precursor for variety of bis(cyclopentadienyl)vanadium(IV) compounds.
Reduction of vanadocene dichloride gives vanadocene (C5H5)2V.
Contents
Preparation
Cp2VCl2 was first prepared by Wilkinson and Birmingham via the reaction of NaC5H5 and VCl4 in THF, followed by work up by extraction with chloroform and hydrogen chloride and recrystallization from toluene.[1]
Structure
Like titanocene dichloride, this organovanadium compound is currently being investigated as a potential anticancer drug (currently in clinical trials). The mechanism by which it acts is not understood, but some conjecture that it might be due to its interactions with the protein transferrin.[2]
References
- ^ G. Wilkinson and J.G. Birmingham (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". Journal of the American Chemical Society 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- ^ Murthy M. S., Rao L. N., Kuo L. Y., Toney J. H., Marks T. J. (1988). "Antitumor and toxicologic properties of the organometallic anticancer agent vanadocene dichloride.". Inorg. Chimica Acta 152 (2): 117–124. doi:10.1016/S0020-1693(00)83343-5.
Further reading
- T. Hirao, A. Ogawa, M. Asahara, Y. Muguruma, H. Sakurai (2005), "d,l-Selective Pinacol-Type Coupling Using Zinc, Chlorosilane, and Catalytic Amount of Cp2VCl2; dl-1,2-Dicyclohexylethanediol", Org. Synth. 81: 26, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v81p0026
Categories:- Chlorides
- Metallocenes
- Organovanadium compounds
Wikimedia Foundation. 2010.

