- Pristane
-
Pristane 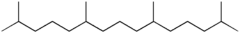
 2,6,10,14-TetramethylpentadecaneOther namesNorphytane
2,6,10,14-TetramethylpentadecaneOther namesNorphytaneIdentifiers CAS number 1921-70-6 
PubChem 15979 ChemSpider 15182 
ChEBI CHEBI:53181 
Jmol-3D images Image 1 - C(CCCC(CCCC(C)CCCC(C)C)C)(C)C
Properties Molecular formula C19H40 Molar mass 268.51 g/mol Density 0.783 g/cm3 Melting point -100 °C, 173 K, -148 °F
Boiling point 296 °C, 569 K, 565 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Pristane is a natural saturated terpenoid alkane obtained primarily from shark liver oil, from which its name is derived (Latin pristis, "shark"). It is also found in mineral oil and some foods.[1] It is a transparent oily liquid that is immiscible with water, but soluble in diethyl ether, benzene, chloroform and carbon tetrachloride.
Pristane is known to induce autoimmune diseases in rodents. It is used in research to understand the pathogenesis of rheumatoid arthritis and lupus.[2][3] The fact that it is used in many products, raises the possibility that it may be a possible environmental exposure that may trigger diseases such as lupus and rheumatoid arthritis.[4]
It is used as a lubricant, a transformer oil, an immunologic adjuvant, and an anti-corrosion agent, biological marker, plasmocytomas inducer and in production of monoclonal antibodies.
Biosynthetically, pristane is derived from phytol. Often used as a biomarker in petroleum studies[5].
References
- ^ Chung, J. G., L. R. Garrett, P. E. Byers, and M. A. Cuchens (1989). "A survey of the amount of pristane in common fruits and vegetables". J. Food Comp. Anal. 2 (22): 22. doi:10.1016/0889-1575(89)90058-6.
- ^ Anderson, P. N., and M. Potter (1969). "Induction of plasma cell tunours in BALBfc mice with 2,6,10,14-tetramethylpentadecane (pristane)". Nature 222 (5197): 994. doi:10.1038/222994a0. PMID 5789334.
- ^ Hazani R, Engineer N. (Nov 2008). "Surreptitious injection of mineral oil: a case report of sclerosing lipogranulomatosis". Ann Plast Surg 61 (5): 555–8. doi:10.1097/SAP.0b013e31816d8316. PMID 18948786.
- ^ Frederick W Miller (2006). "Is occupational exposure to mineral oil a risk factor for rheumatoid arthritis?". Nature Clinical Practice Rheumatology 2 (3): 130–131. doi:10.1038/ncprheum0137. PMID 16932671.
- ^ Hunt, J. (2002). "Early developments in petroleum geochemistry". Organic Geochemistry 33: 1025–1052. doi:10.1016/S0146-6380(02)00056-6.
Categories:- Alkanes
- Diterpenes
Wikimedia Foundation. 2010.
