- Triuranium octoxide
-
Triuranium octoxide 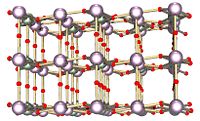 Other namespitchblende
Other namespitchblendeIdentifiers CAS number 1317-99-3 Properties Molecular formula U3O8 Molar mass 842.1 g/mol Melting point 1150°C
Boiling point decomposes to UO2 at 1300 °C
Solubility in other solvents Insoluble in water; Soluble in nitric and sulfuric acids.
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Triuranium octoxide (U3O8) is a compound of uranium. It is present as an olive green to black, odorless solid. In spite of its color, it is one of the more popular forms of yellowcake and is shipped between mills and refineries in this form.
Triuranium octoxide occurs naturally as the olive-green-colored mineral pitchblende. U3O8 is readily produced from UF6 and has potential long-term stability in a geologic environment. In the presence of oxygen (O2), uranium dioxide (UO2) is oxidized to U3O8, whereas uranium trioxide (UO3) loses oxygen at temperatures above 500°C and is reduced to U3O8. The compound can be produced by any one of three primary chemical conversion processes, involving either uranium tetrafluoride (UF4) or uranyl fluoride (UO2F2) as intermediates. It is generally considered to be the more attractive form for disposal purposes because, under normal environmental conditions, U3O8 is one of the most kinetically and thermodynamically stable forms of uranium and also because it is the form of uranium found in nature. Its particle density is 8.3 g cm−3.
Solid state structure
The solid is a layered structure where the layers are bridged by oxygen atoms, each layer contains uranium atoms which are in different coordination environments in the above diagram these are shown in plum and green.
Bond valence study
Using a 6Å x 6Å x 6Å box with the uranium atom in the centre the bond valence calculation was performed for both U1 and U2 in solid.[1] It was found using the parameters for U(VI) that the calculated oxidation states for U1 and U2 are 5.11 and 5.10. Using the parameters for U(IV) the calculated oxidation states are 5.78 and 5.77 respectively for U1 and U2. These study suggests that all the uranium atoms have the same oxidation state, so that the oxidation states are disordered through the lattice.
References
Uranium compounds (NH4)2U2O7 · Na2O(UO3)2 · Na2U2O7 · UB2 · U(BH4)4 · UCl3 · UCl4 · UF4 · UF5 · UF6 · UH3 · UO2 · UO3 · UO4 · U3O8 · UO2(CH3COO)2 · UO2(CHO2)2 · UO2CO3 · UO2CO3·2(NH4)2CO3 · UO2Cl2 · UO2F2 · UO2(NO3)2 · UO2(OH)2 · UO2(SO4)2 · U(S04)2 · ZnUO2(CH3COO)4
Categories:- Uranium compounds
- Oxides
- Nuclear materials
- Inorganic compound stubs
Wikimedia Foundation. 2010.
