- 1,2-Benzoquinone
-
1,2-Benzoquinone 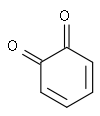
 cyclohexa-3,5-diene-1,2-dioneOther names1,2-benzoquinone, o-benzoquinone, o-quinone
cyclohexa-3,5-diene-1,2-dioneOther names1,2-benzoquinone, o-benzoquinone, o-quinoneIdentifiers CAS number 583-63-1 PubChem 11421 ChemSpider 10941 
UNII SVD1LJ47R7 
KEGG C02351 
ChEBI CHEBI:17253 
Jmol-3D images Image 1
Image 2- O=C1/C=C\C=C/C1=O
C1=CC(=O)C(=O)C=C1
Properties Molecular formula C6H4O2 Molar mass 108.0964 g/mol Density 1.256 g/cm3 Boiling point 213.3 °C @760 mmHg
Hazards Flash point 76.4 °C Related compounds Related compounds 1,4-benzoquinone quinone  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,2-Benzoquinone, also called ortho-benzoquinone or cyclohexa-3,5-diene-1,2-dione, is a ketone, with formula C6H4O2. It is one of the two isomers of quinone, the other being 1,4-benzoquinone.
1,2-Benzoquinone is produced on oxidation of catechol exposed to air in aqueous solution[1][2] or by ortho oxidation of a phenol.[1] It is a precursor to melanin.[3] It is a red substance soluble in water and insoluble in ethyl ether.
A strain of the bacterium Pseudomonas mendocina metabolyzes benzoic acid yielding 1,2-benzoquinone (via cathecol) as the final product.[2]
See also
- 1,4-Benzoquinone
- Tetrabromo-1,2-benzoquinone (o-bromanil)
- Tetrachloro-1,2-benzoquinone (o-chloranil)
- Tetrahydroxy-1,2-benzoquinone
- Hydroxybenzoquinone
References
- ^ a b Magdziak, D., Rodriguez, A. A.; Van De Water, R. W.; Pettus, T. R. R. (2002). "Regioselective oxidation of phenols to o-quinones with o-iodoxybenzoic acid (IBX)". Org. Lett. 4 (2): 285–288. doi:10.1021/ol017068j. PMC 1557836. PMID 11796071. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1557836.
- ^ a b Chanda Parulekar and Suneela Mavinkurve (2006), Formation of ortho-benzoquinone from sodium benzoate by Pseudomonas mendocina P2d. Indian Journal of Experimental Biology, volume 44, pages 157--162. Online version accessed on 2010-02-04.
- ^ Enzymatic Browning in Fruits, Vegetables and Seafoods Section 2.3.2
This article about a ketone is a stub. You can help Wikipedia by expanding it. - O=C1/C=C\C=C/C1=O
