- Dihydrouridine
-
Dihydrouridine 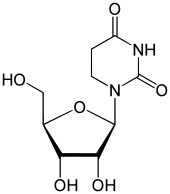 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-diazinane-2,4-dioneOther names3,4-dihydrouridine
1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-diazinane-2,4-dioneOther names3,4-dihydrouridine
3,4,5,6-tetrahydrouridine
5,6-dihydrouridineIdentifiers CAS number 5627-05-4 PubChem 94312 Jmol-3D images Image 1 - C1CN(C(=O)NC1=O)[C@H]2[C@@H]([C@@H]([C@H](O2)CO)O)O
Properties Molecular formula C9H14N2O6 Molar mass 246.217 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dihydrouridine (abbreviated as D, DHU, or UH2) is a pyrimidine which is the result of adding two hydrogen atoms to a uridine, making it a fully saturated pyrimidine ring with no remaining double bonds. D is found in tRNA and rRNA molecules as a nucleoside; the corresponding nucleobase is 5,6-Dihydrouracil.
Because it is non-planar, D disturbs the stacking interactions in helices and destabilizes the RNA structure. D also stabilizes the C2’-endo sugar conformation, which is more flexible then the C3’-endo conformation, and this effect is propagated to the 5’-neighboring residue. Thus, while pseudouridine and 2’-O-methylations stabilize the local RNA structure, D does the opposite.[1]
tRNA of organisms that grow at low temperatures (psychrophiles) have high 5,6-dihydrouridine levels (40-70% more on average) which provides the necessary, local, flexibility of the tRNA at or below the freezing point.[2]
See also
References
- ^
 Dalluge JJ; Hashizume T, Sopchik AE, McCloskey JA, Davis DR. (Mar 15 1996). "Conformational flexibility in RNA: the role of dihydrouridine". Nucleic Acids Res 24 (6): 1073–1079. doi:10.1093/nar/24.6.1073. PMC 145759. PMID 8604341. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=145759.
Dalluge JJ; Hashizume T, Sopchik AE, McCloskey JA, Davis DR. (Mar 15 1996). "Conformational flexibility in RNA: the role of dihydrouridine". Nucleic Acids Res 24 (6): 1073–1079. doi:10.1093/nar/24.6.1073. PMC 145759. PMID 8604341. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=145759. - ^
 Dalluge JJ; Hamamoto T, Horikoshi K, Morita RY, Stetter KO, and McCloskey JA (March 1 1997). "Posttranscriptional modification of tRNA in psychrophilic bacteria". J Bacteriol 179 (6): 1918–1923. PMC 178914. PMID 9068636. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=178914.
Dalluge JJ; Hamamoto T, Horikoshi K, Morita RY, Stetter KO, and McCloskey JA (March 1 1997). "Posttranscriptional modification of tRNA in psychrophilic bacteria". J Bacteriol 179 (6): 1918–1923. PMC 178914. PMID 9068636. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=178914.
Categories:- Nucleosides
- Pyrimidinediones
- Ribosides
Wikimedia Foundation. 2010.
