- Pyruvate dehydrogenase (lipoamide) alpha 1
-
Pyruvate dehydrogenase (lipoamide) alpha 1 
PDB rendering based on 1ni4.Available structures PDB 1NI4, 2OZL, 3EXE, 3EXF, 3EXG, 3EXH, 3EXI Identifiers Symbols PDHA1; PDHA; PDHCE1A; PHE1A External IDs OMIM: 300502 MGI: 97532 HomoloGene: 121491 GeneCards: PDHA1 Gene EC number 1.2.4.1 Gene Ontology Molecular function • pyruvate dehydrogenase (acetyl-transferring) activity
• protein binding
• oxidoreductase activityCellular component • mitochondrion
• mitochondrial matrixBiological process • pyruvate metabolic process
• glycolysis
• regulation of acetyl-CoA biosynthetic process from pyruvateSources: Amigo / QuickGO RNA expression pattern 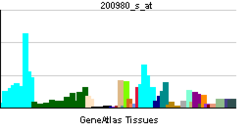
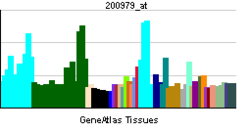
More reference expression data Orthologs Species Human Mouse Entrez 5160 18597 Ensembl ENSG00000131828 ENSMUSG00000031299 UniProt P08559 Q3UFJ3 RefSeq (mRNA) NM_000284.3 NM_008810.2 RefSeq (protein) NP_000275.1 NP_032836.1 Location (UCSC) Chr X:
19.36 – 19.38 MbChr X:
156.56 – 156.58 MbPubMed search [1] [2] Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial is an enzyme that in humans is encoded by the PDHA1 gene.
The pyruvate dehydrogenase complex is a nuclear-encoded mitochondrial matrix multienzyme complex that provides the primary link between glycolysis and the tricarboxylic acid (TCA) cycle by catalyzing the irreversible conversion of pyruvate into acetyl-CoA. The PDH complex is composed of multiple copies of 3 enzymes: E1 (PDHA1); dihydrolipoyl transacetylase (DLAT; MIM 608770) (E2; EC 2.3.1.12); and dihydrolipoyl dehydrogenase (DLD; MIM 238331) (E3; EC 1.8.1.4). The E1 enzyme is a heterotetramer of 2 alpha and 2 beta subunits. The E1-alpha subunit contains the E1 active site and plays a key role in the function of the PDH complex (Brown et al., 1994).[supplied by OMIM][1]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[2]
Citric_acid_cycle edit
References
- ^ "Entrez Gene: PDHA1 pyruvate dehydrogenase (lipoamide) alpha 1". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5160.
- ^ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78". http://www.wikipathways.org/index.php/Pathway:WP78.
Further reading
- Dahl HH, Brown GK, Brown RM, et al. (1993). "Mutations and polymorphisms in the pyruvate dehydrogenase E1 alpha gene.". Hum. Mutat. 1 (2): 97–102. doi:10.1002/humu.1380010203. PMID 1301207.
- Brown GK, Otero LJ, LeGris M, Brown RM (1995). "Pyruvate dehydrogenase deficiency.". J. Med. Genet. 31 (11): 875–9. doi:10.1136/jmg.31.11.875. PMC 1016663. PMID 7853374. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1016663.
- Sugden MC, Holness MJ (2003). "Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs.". Am. J. Physiol. Endocrinol. Metab. 284 (5): E855–62. doi:10.1152/ajpendo.00526.2002. PMID 12676647.
- Dahl HH, Hansen LL, Brown RM, et al. (1993). "X-linked pyruvate dehydrogenase E1 alpha subunit deficiency in heterozygous females: variable manifestation of the same mutation.". J. Inherit. Metab. Dis. 15 (6): 835–47. doi:10.1007/BF01800219. PMID 1293379.
- Ito M, Huq AH, Naito E, et al. (1993). "Mutation of E1 alpha gene in a female patient with pyruvate dehydrogenase deficiency due to rapid degradation of E1 protein.". J. Inherit. Metab. Dis. 15 (6): 848–56. doi:10.1007/BF01800220. PMID 1338114.
- De Meirleir L, Lissens W, Vamos E, Liebaers I (1992). "Pyruvate dehydrogenase (PDH) deficiency caused by a 21-base pair insertion mutation in the E1 alpha subunit.". Hum. Genet. 88 (6): 649–52. doi:10.1007/BF02265291. PMID 1551669.
- Hansen LL, Brown GK, Kirby DM, Dahl HH (1991). "Characterization of the mutations in three patients with pyruvate dehydrogenase E1 alpha deficiency.". J. Inherit. Metab. Dis. 14 (2): 140–51. doi:10.1007/BF01800586. PMID 1909401.
- Koike K, Urata Y, Matsuo S, Koike M (1990). "Characterization and nucleotide sequence of the gene encoding the human pyruvate dehydrogenase alpha-subunit.". Gene 93 (2): 307–11. doi:10.1016/0378-1119(90)90241-I. PMID 2227443.
- Endo H, Hasegawa K, Narisawa K, et al. (1989). "Defective gene in lactic acidosis: abnormal pyruvate dehydrogenase E1 alpha-subunit caused by a frame shift.". Am. J. Hum. Genet. 44 (3): 358–64. PMID 2537010.
- Brown RM, Dahl HH, Brown GK (1989). "X-chromosome localization of the functional gene for the E1 alpha subunit of the human pyruvate dehydrogenase complex.". Genomics 4 (2): 174–81. doi:10.1016/0888-7543(89)90297-8. PMID 2737678.
- Maragos C, Hutchison WM, Hayasaka K, et al. (1989). "Structural organization of the gene for the E1 alpha subunit of the human pyruvate dehydrogenase complex.". J. Biol. Chem. 264 (21): 12294–8. PMID 2745444.
- Ho L, Wexler ID, Liu TC, et al. (1989). "Characterization of cDNAs encoding human pyruvate dehydrogenase alpha subunit.". Proc. Natl. Acad. Sci. U.S.A. 86 (14): 5330–4. doi:10.1073/pnas.86.14.5330. PMID 2748588.
- De Meirleir L, MacKay N, Lam Hon Wah AM, Robinson BH (1988). "Isolation of a full-length complementary DNA coding for human E1 alpha subunit of the pyruvate dehydrogenase complex.". J. Biol. Chem. 263 (4): 1991–5. PMID 2828359.
- Dahl HH, Hunt SM, Hutchison WM, Brown GK (1987). "The human pyruvate dehydrogenase complex. Isolation of cDNA clones for the E1 alpha subunit, sequence analysis, and characterization of the mRNA.". J. Biol. Chem. 262 (15): 7398–403. PMID 3034892.
- Koike K, Ohta S, Urata Y, et al. (1988). "Cloning and sequencing of cDNAs encoding alpha and beta subunits of human pyruvate dehydrogenase.". Proc. Natl. Acad. Sci. U.S.A. 85 (1): 41–5. doi:10.1073/pnas.85.1.41. PMID 3422424.
- Hansen LL, Horn N, Dahl HH, Kruse TA (1994). "Pyruvate dehydrogenase deficiency caused by a 33 base pair duplication in the PDH E1 alpha subunit.". Hum. Mol. Genet. 3 (6): 1021–2. doi:10.1093/hmg/3.6.1021. PMID 7545958.
- Takakubo F, Cartwright P, Hoogenraad N, et al. (1995). "An amino acid substitution in the pyruvate dehydrogenase E1 alpha gene, affecting mitochondrial import of the precursor protein.". Am. J. Hum. Genet. 57 (4): 772–80. PMID 7573035.
- Hemalatha SG, Kerr DS, Wexler ID, et al. (1995). "Pyruvate dehydrogenase complex deficiency due to a point mutation (P188L) within the thiamine pyrophosphate binding loop of the E1 alpha subunit.". Hum. Mol. Genet. 4 (2): 315–8. doi:10.1093/hmg/4.2.315. PMID 7757088.
PDB gallery Categories:- Human proteins
- Chromosome X gene stubs
Wikimedia Foundation. 2010.



