- Neptunium(IV) oxide
-
Neptunium(IV) oxide 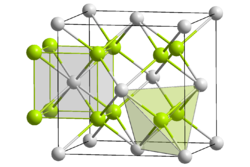 Neptunium(IV) oxideOther namesNeptunium oxide, Neptunium dioxide
Neptunium(IV) oxideOther namesNeptunium oxide, Neptunium dioxideIdentifiers CAS number 12035-79-9 Properties Molecular formula NpO2 Molar mass 269 g/mol Appearance Green cubic crystals Density 11.1 g/cm3 Melting point 2547 °C[1]
Structure Crystal structure cubic crystal system, cF12 Space group Fm3m, #225 Coordination
geometryNp, 8, cubic
O, 4, tetrahedralThermochemistry Std enthalpy of
formation ΔfHo298−256.7 ± 0.6 kcal·mol-1
(−1074 ± 3 kJ·mol-1)[2]Standard molar
entropy So29819.19 ± 0.1 cal·mol-1·K-1
(80.3 ± 0.4 J·mol-1·K-1)[3]Related compounds Other anions Neptunium(III) chloride
Neptunium(IV) chlorideOther cations Uranium(VI) oxide
Plutonium(IV) oxide
Promethium(III) oxide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Neptunium(IV) oxide or neptunium dioxide is the chemical compound composed of neptunium and oxygen with the formula NpO2. It forms dark olive[4] green cubic crystals[1].
Uses
Metal targets which will later be irradiated by plutonium are formed of neptunium(IV) oxide.[5].
Reactions
Neptunium(IV) oxide is formed when neptunium(IV) oxalate or neptunium(IV) peroxide is precipitated from aqueous solution and then calcined in air.[6]
References
- ^ a b Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). CRC Press. pp. 471. ISBN 0-84930594-2.
- ^ Huber, Jr., Elmer J.; Charles E. Holley, Jr. (October 1968). "Enthalpy of formation of neptunium dioxide". Journal of Chemical Engineering Data 13 (4): 545–546. doi:10.1021/je60039a029.
- ^ Westrum, Jr., Edgar F.; J. B. Hatcher, Darrell W. Osborne (March 1953). "The Entropy and Low Temperature Heat Capacity of Neptunium Dioxide". Journal of Chemical Physics 21 (3): 419. doi:10.1063/1.1698923.
- ^ Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill Professional. pp. 271. ISBN 0-07049439-8. http://www.google.com/books?id=0fT4wfhF1AsC&pg=PA271&dq=%22neptunium(IV)+oxide%22+OR+%22neptunium+dioxide%22&as_brr=3&ei=MaDBScOjBpK8zATZtPHGBA#PPA271,M1. Retrieved 2009-03-18.
- ^ Krebs, Robert E. (2006). The History and Use of our Earth's Chemical Elements. Greenwood Publishing Group. pp. 318. ISBN 978-0-31333438-2. http://www.google.com/books?id=yb9xTj72vNAC&pg=PA318&dq=%22neptunium(IV)+oxide%22+OR+%22neptunium+dioxide%22&as_brr=3&ei=MaDBScOjBpK8zATZtPHGBA. Retrieved 2009-03-18.
- ^ Porter, J. A. (October 1964). "Production of Neptunium Dioxide". Industrial and Engineering Chemistry Process Design and Development 3 (4): 289–292. doi:10.1021/i260012a001.
Neptunium compounds NpO2
Categories:- Inorganic compound stubs
- Neptunium compounds
- Oxides
Wikimedia Foundation. 2010.
