- Diphenyldichloromethane
-
Diphenyldichloromethane 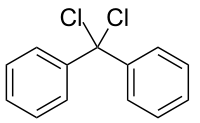 diphenyldichloromethaneOther namesbis-chlorodiphenylmethane; bisphenyldichloromethane; diphenyl-dichloromethane; dichlorodiphenylmethane; α,α-dichlorodiphenylmethane; 1,1-dichloro-1,1-diphenylmethane; 1,1-dichlorodiphenylmethane
diphenyldichloromethaneOther namesbis-chlorodiphenylmethane; bisphenyldichloromethane; diphenyl-dichloromethane; dichlorodiphenylmethane; α,α-dichlorodiphenylmethane; 1,1-dichloro-1,1-diphenylmethane; 1,1-dichlorodiphenylmethaneIdentifiers CAS number 2051-90-3 PubChem 16327 ChemSpider 15492 
Beilstein Reference 1910601 Jmol-3D images Image 1 - c1ccc(cc1)C(c2ccccc2)(Cl)Cl
Properties Molecular formula C13H10Cl2 Molar mass 237.12 g mol−1 Appearance clear light yellow liquid Density 1.235 g/cm3 Melting point 146 - 150 °C[1]
Boiling point 193 °C at 32 torr[2]
Hazards Flash point 110 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diphenyldichloromethane is used in the synthesis of tetraphenylethylene[3]
Synthesis
Benzophenone is reacted with phosphorus pentachloride to give diphenyldichloromethane[4]
References
- ^ Ballester, Manuel; Juan Riera-Figueras, Juan Castaner, Carlos Badfa, Jose M. Monso (1971). "Inert carbon free radicals. I. Perchlorodiphenylmethyl and perchlorotriphenylmethyl radical series". Journal of the American Chemical Society 93 (9): 2215–2225. doi:10.1021/ja00738a021. ISSN 0002-7863.
- ^ Andrews, L. J.; W. W. Kaeding (1951). "The Formation of Benzophenone and its Diethylketal in the Ethanolysis of Diphenyldichloromethane". Journal of the American Chemical Society 73 (3): 1007–1011. doi:10.1021/ja01147a036. ISSN 0002-7863.
- ^ Inaba, S (1982). "Metallic nickel as a reagent for the coupling of aromatic and benzylic halides". Tetrahedron Letters 23 (41): 4215–4216. doi:10.1016/S0040-4039(00)88707-9. ISSN 00404039.
- ^ Spaggiari, Alberto; Daniele Vaccari, Paolo Davoli, Giovanni Torre, Fabio Prati (2007). "A Mild Synthesis of Vinyl Halides andgem-Dihalides Using Triphenyl Phosphite−Halogen-Based Reagents". The Journal of Organic Chemistry 72 (6): 2216–2219. doi:10.1021/jo061346g. ISSN 0022-3263. PMID 17295542.

This article about an organic halide is a stub. You can help Wikipedia by expanding it.
