- Transition metal dinitrogen complex
-
Metal dinitrogen complexes are a coordination compounds that contain the dinitrogen (N2) as a ligand. In the area of coordination chemistry, the atomic and diatomic forms of nitrogen are distinguished, although otherwise "nitrogen" refers to N2.
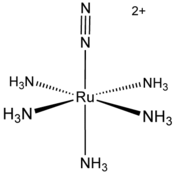
Metal complexes of N2 have been studied since 1965 when the first complex was reported by Allen and Senoff. This complex, [Ru(NH3)5(N2)]2+consists of a [Ru(NH3)5]2+ centre attached to one end of N2.[1] Interest in such complexes arises because N2 comprises the majority of the atmosphere and because many useful compounds contain nitrogen atoms. Biological nitrogen fixation probably occurs via the binding of N2 to a metal center in the enzyme nitrogenase, followed by a series of steps that involve electron transfer and protonation. The hydrogenation of N2 is only weakly exothermic, hence the industrial hydrogenation of nitrogen via the Haber-Bosch Process employs high pressures and high temperatures.[2]
Contents
Bonding modes
In terms of its bonding to transition metals, N2 is related to CO and acetylene as all three species have triple bonds. A variety of bonding modes have been characterized.
End-on
As a ligand, N2 usually binds to metals as an "end-on" ligand, as illustrated by Allen and Senoff's complex. Such complexes are usually analgous to related CO derivatives. A good example of this relationship are the complexes IrCl(CO)(PPh3)2 and IrCl(N2)(PPh3)2.[3] Few complexes contain more than one N2 ligand, and no example features three (in contrast metal hexacarbonyls are common). The dinitrogen ligand in W(N2)2(Ph2CH2CH2PPh2)2 can be reduced to produce ammonia.[4]
Bridging, end-on
N2 also serves as a bridging ligand, as illustrated by {[Ru(NH3)5]2(μ-N2)}4+.
A study in 2006 of iron-dinitrogen complexes showed that the N–N bond is significantly weakened upon complexation with iron atoms with a low coordination number. The complex involved bidentate chelating ligands attached to the iron atoms in the Fe–N–N–Fe core, in which N2 acts as a bridging ligand between the iron atoms. Increasing the coordination number of iron by modifying the chelating ligands and adding an additional ligand per iron atom showed an increase in the strength of the N–N bond in the resulting complex. It is thus suspected that Fe in a low-coordination environment is a key factor to the fixation of nitrogen by the nitrogenase enzyme, since its Fe–Mo cofactor also features Fe with low coordination numbers.[5]
Side-on, bridging
In a second mode of bridging, bimetallic complexes are known wherein the N-N vector is perpendicular to the M-M vector. One example is [(η5-C5Me4H)2Zr]2(μ2,η2,η2-N2).[6]
References
- ^ A. D. Allen, C. V. Senoff (1965). "Nitrogenopentammineruthenium(II) complexes". Journal of the Chemical Society, Chemical Communications (24): 621. doi:10.1039/C19650000621.
- ^ Fryzuk, M. D.: Johnson, S. A (2000). "The continuing story of dinitrogen activation". Coordination Chemistry Reviews 200–202: 379. doi:10.1016/S0010-8545(00)00264-2.
- ^ Collman, J. P.; Hoffman, N. W.; Hosking, J. W. (2000). "trans-Chloro(nitrogen)bis(triphenylphosphine)iridium (I)". Inorganic Syntheses 12: 8–11. ISBN 9780470131718.
- ^ Modern Coordination Chemistry: The Legacy of Joseph Chatt” G. J. Leigh (Editor), N. W. Winterton (Editor) Springer Verlag (2002). ISBN 0854044698
- ^ Smith, J.; Sadique, A.; Cundari, T.; Rodgers, K.; Lukat-Rodgers, G.; Lachicotte, R.; Flaschenriem, C.; Vela, J. et al. (2006). "Studies of low-coordinate iron dinitrogen complexes". Journal of the American Chemical Society 128 (3): 756–769. doi:10.1021/ja052707x. PMID 16417365.
- ^ Bernskoetter, W. H.; Lobkovsky, E.; Chirik, P. J. (2005). "Kinetics and Mechanism of N2 Hydrogenation in Bis(cyclopentadienyl) Zirconium Complexes and Dinitrogen Functionalization by 1,2-Addition of a Saturated C-H Bond". Journal of the American Chemical Society 127 (40): 14051–14061. doi:10.1021/ja0538841. PMID 16201827.
Categories:- Nitrogen compounds
- Organometallic chemistry
Wikimedia Foundation. 2010.