- 2,6-Diaminopurine
-
2,6-Diaminopurine 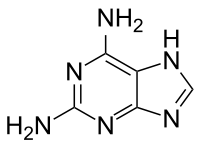 7H-Purine-2,6-diamineOther names2-Aminoadenine
7H-Purine-2,6-diamineOther names2-AminoadenineIdentifiers CAS number 1904-98-9 
PubChem 30976 ChemSpider 28738 
ChEMBL CHEMBL388596  =
=Jmol-3D images Image 1 - c1[nH]c2c(nc(nc2n1)N)N
Properties Molecular formula C5H6N6 Molar mass 150.14 g mol−1 Appearance White to yellow crystalline powder Density 1.743 g/cm3 Melting point 117-122 °C
Solubility in water 2.38 g/L @ 20°C 
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2,6-Diaminopurine is a compound used to treat leukemia.[citation needed]
In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting 2,6-diaminopurine and related organic molecules, including the DNA and RNA components adenine and guanine, may have been formed extraterrestrially in outer space.[1][2][3]
Cyanophage S-2L
Main article: nucleic acid analoguesIn Cyanophage S-2L diaminopurine (DAP) is used instead of adenine (host evasion).[4] Diaminopurine basepairs perfectly with thymine as it is identical to adenine but has an amine group at position 2 forming 3 intramolecular hydrogen bonds, eliminating the major difference between the two types of basepairs (Weak:A-T and Strong:C-G). This improved stability affects protein-binding interactions that rely on those differences.
References
- ^ Callahan; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS. doi:10.1073/pnas.1106493108. http://www.pnas.org/content/early/2011/08/10/1106493108. Retrieved 2011-08-15.
- ^ Steigerwald, John (08 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. http://www.nasa.gov/topics/solarsystem/features/dna-meteorites.html. Retrieved 2011-08-10.
- ^ ScienceDaily Staff (09 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. http://www.sciencedaily.com/releases/2011/08/110808220659.htm. Retrieved 2011-08-09.
- ^ Kirnos MD, Khudyakov IY, Alexandrushkina NI, Vanyushin BF. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977 Nov 24;270(5635):369–70.
This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it.
