- DHHC domain
-
DHHC domain 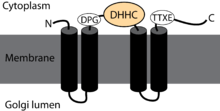
A depiction of the topology of DHHC family palmitoyltransferases. Transmembrane alpha helices are represented as black tubes. The DHHC domain is shown as a light orange oval. Identifiers Symbol DHHC Pfam PF01529 InterPro IPR001594 PROSITE PDOC50216 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary In molecular biology the DHHC domain is a protein domain that acts as an enzyme, which adds a palmitoyl chemical group to proteins in order to anchor them to cell membranes. The DHHC domain was discovered in 1999 and named after a conserved sequence motif found in its protein sequence.[1] Roth and colleagues showed that the yeast Akr1p protein could palmitoylate Yck2p in vitro and inferred that the DHHC domain defined a large family of palmitoyltransferases.[2] In mammals twenty three members of this family have been identified and their substrate specificities investigated.[3] Some members of the family such as ZDHHC3 and ZDHHC7 enhance palmitoylation of proteins such as PSD-95, SNAP-25, GAP43, Gαs. Others such as ZDHHC9 showed specificity only toward the H-Ras protein.[3] However, a recent study questions the involvement of classical enzyme-substrate recognition and specificity in the palmitoylation reaction.[4] Several members of the family have been implicated in human diseases.
Contents
Sequence motifs
Conserved motifs within protein sequences point towards the most important amino acid residues for function. In the DHHC domain there is a tetrapeptide motif composed of aspartate-histidine-histidine-cysteine. However this short sequence is embedded in a larger region of about fifty amino acids in length that shares many more conserved amino acids. The canonical DHHC domain can be described with the following sequence motif:
C-x2-C-x9-HC-x2-C-x4-DHHC-x5-C-x4-N-x3-F (x shows region of unconserved residues) However many examples of DHHC domains are known that do not contain all these conserved residues. In addition to the central DHHC domain three further sequence motifs have been identified in members of the DHHC family. A DPG (aspartate-proline-glycine) motif has been identified just to the C-terminus of the second transmembrane region.[5] A TTxE (threonine-threonine-any-glutamate) motif has also been identified after the fourth transmembrane helix.[5] A third motif towards the C-terminus of many proteins has been identified that contains a conserved aromatic amino acid, a glycine and an asparagine.[6]
Chemical inhibitors
In 2006, five chemical classes of small molecules were discovered which were shown to act against palmitoyltransferases.[7] Further studies in 2009 showed that of the 5 classes studied, 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one was shown to behave similarly to 2-Bromopalmitate and were identified as able to inhibit the palmitoylation reaction of a range of DHHC domain containing proteins. Inhibition with 2-Bromopalmitate was found to be irreversible, the other however was found to be mostly reversible.[8] Because of the roles of DHHC domain proteins in human diseases it has been suggested that chemical inhibitors of specific DHHC proteins may be a potential route to treatment of disease.[8]
In human disease
Several proteins containing DHHC domains have been implicated in human disease. Two missense mutations within the DHHC domain of ZDHHC9 were identified in X-linked mental retardation associated with a Marfanoid Habitus.[9] A potential link of ZDHHC11 with bladder cancer has been suggested by the discovery that 5 out of 9 high-grade bladder cancer samples surveyed contained a duplication of the 5p15.33 genomic region.[10] However, this region contains another gene TPPP which may be the causative gene. The HIP14 palmitoyltransferase is responsible for palmitoylating the Huntingtin protein. Expansions of the triplet repeat in the huntington's gene leads to loss of interaction with HIP14 which Yanai and colleagues speculate is involved in the pathology of Huntington's disease.[11] A gene knockout experiment of the mouse homologue of ZDHHC13 showed hair loss, severe osteoporosis, and systemic amyloidosis, both of AL and AA depositions.[12]
See also
References
- ^ Putilina T, Wong P, Gentleman S (May 1999). "The DHHC domain: a new highly conserved cysteine-rich motif". Mol. Cell. Biochem. 195 (1-2): 219–26. doi:10.1023/A:1006932522197. PMID 10395086.
- ^ Roth AF, Feng Y, Chen L, Davis NG (October 2002). "The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase". J. Cell Biol. 159 (1): 23–8. doi:10.1083/jcb.200206120. PMC 2173492. PMID 12370247. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2173492.
- ^ a b Fukata Y, Iwanaga T, Fukata M (October 2006). "Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells". Methods 40 (2): 177–82. doi:10.1016/j.ymeth.2006.05.015. PMID 17012030.
- ^ Rocks O, Gerauer M, Vartak N, et al. (April 2010). "The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins". Cell 141 (3): 458–71. doi:10.1016/j.cell.2010.04.007. PMID 20416930.
- ^ a b Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ (June 2006). "Protein palmitoylation by a family of DHHC protein S-acyltransferases". J. Lipid Res. 47 (6): 1118–27. doi:10.1194/jlr.R600007-JLR200. PMID 16582420.
- ^ González Montoro A, Quiroga R, Maccioni HJ, Valdez Taubas J (April 2009). "A novel motif at the C-terminus of palmitoyltransferases is essential for Swf1 and Pfa3 function in vivo". Biochem. J. 419 (2): 301–8. doi:10.1042/BJ20080921. PMID 19138168.
- ^ Stober R (June 1987). "[Total or subtotal amputation of a long finger with destruction of the metacarpophalangeal joint--regaining function by replantation?]" (in German). Aktuelle Traumatol 17 (3): 100–4. PMID 2888271.
- ^ a b Jennings BC, Nadolski MJ, Ling Y, et al. (February 2009). "2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro ". J. Lipid Res. 50 (2): 233–42. doi:10.1194/jlr.M800270-JLR200. PMC 2636914. PMID 18827284. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2636914.
- ^ Raymond FL, Tarpey PS, Edkins S, et al. (May 2007). "Mutations in ZDHHC9, which encodes a palmitoyltransferase of NRAS and HRAS, cause X-linked mental retardation associated with a Marfanoid habitus". Am. J. Hum. Genet. 80 (5): 982–7. doi:10.1086/513609. PMC 1852737. PMID 17436253. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1852737.
- ^ Yamamoto Y, Chochi Y, Matsuyama H, et al. (2007). "Gain of 5p15.33 is associated with progression of bladder cancer". Oncology 72 (1-2): 132–8. doi:10.1159/111132. PMID 18025801.
- ^ Yanai A, Huang K, Kang R, et al. (June 2006). "Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function". Nat. Neurosci. 9 (6): 824–31. doi:10.1038/nn1702. PMC 2279235. PMID 16699508. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2279235.
- ^ Saleem AN, Chen YH, Baek HJ, et al. (2010). "Mice with alopecia, osteoporosis, and systemic amyloidosis due to mutation in Zdhhc13, a gene coding for palmitoyl acyltransferase". PLoS Genet. 6 (6): e1000985. doi:10.1371/journal.pgen.1000985. PMC 2883605. PMID 20548961. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2883605.
External Links
- Eukaryotic Linear Motif resource motif class MOD_SPalmitoyl_2
Further reading
- Greaves J, Gorleku OA, Salaun C, Chamberlain LH (August 2010). "Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases". J. Biol. Chem. 285 (32): 24629–38. doi:10.1074/jbc.M110.119289. PMC 2915699. PMID 20519516. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2915699.
- Greaves J, Chamberlain LH (April 2010). "S-acylation by the DHHC protein family". Biochem. Soc. Trans. 38 (2): 522–4. doi:10.1042/BST0380522. PMID 20298214.
- Hines RM, Kang R, Goytain A, Quamme GA (February 2010). "Golgi-specific DHHC zinc finger protein GODZ mediates membrane Ca2+ transport". J. Biol. Chem. 285 (7): 4621–8. doi:10.1074/jbc.M109.069849. PMC 2836067. PMID 19955568. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2836067.
- Mizumaru C, Saito Y, Ishikawa T, et al. (December 2009). "Suppression of APP-containing vesicle trafficking and production of beta-amyloid by AID/DHHC-12 protein". J. Neurochem. 111 (5): 1213–24. doi:10.1111/j.1471-4159.2009.06399.x. PMID 19780898.
- Noritake J, Fukata Y, Iwanaga T, et al. (July 2009). "Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95". J. Cell Biol. 186 (1): 147–60. doi:10.1083/jcb.200903101. PMC 2712995. PMID 19596852. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2712995.
- Hou H, John Peter AT, Meiringer C, Subramanian K, Ungermann C (August 2009). "Analysis of DHHC acyltransferases implies overlapping substrate specificity and a two-step reaction mechanism". Traffic 10 (8): 1061–73. doi:10.1111/j.1600-0854.2009.00925.x. PMID 19453970.
- Greaves J, Prescott GR, Fukata Y, Fukata M, Salaun C, Chamberlain LH (March 2009). "The hydrophobic cysteine-rich domain of SNAP25 couples with downstream residues to mediate membrane interactions and recognition by DHHC palmitoyl transferases". Mol. Biol. Cell 20 (6): 1845–54. doi:10.1091/mbc.E08-09-0944. PMC 2655257. PMID 19158383. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2655257.
- Johswich A, Kraft B, Wuhrer M, et al. (January 2009). "Golgi targeting of Drosophila melanogaster beta4GalNAcTB requires a DHHC protein family-related protein as a pilot". J. Cell Biol. 184 (1): 173–83. doi:10.1083/jcb.200801071. PMC 2615082. PMID 19139268. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2615082.
- Matakatsu H, Blair SS (September 2008). "The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity". Curr. Biol. 18 (18): 1390–5. doi:10.1016/j.cub.2008.07.067. PMC 2597019. PMID 18804377. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2597019.
- Bannan BA, Van Etten J, Kholer JA, et al. (2008). "The Drosophila protein palmitoylome: characterizing palmitoyl-thioesterases and DHHC palmitoyl-transferases". Fly (Austin) 2 (4): 198–214. PMC 2898910. PMID 18719403. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2898910.
- Dighe SA, Kozminski KG (October 2008). "Swf1p, a member of the DHHC-CRD family of palmitoyltransferases, regulates the actin cytoskeleton and polarized secretion independently of its DHHC motif". Mol. Biol. Cell 19 (10): 4454–68. doi:10.1091/mbc.E08-03-0252. PMC 2555925. PMID 18701706. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2555925.
- Lam KK, Davey M, Sun B, Roth AF, Davis NG, Conibear E (July 2006). "Palmitoylation by the DHHC protein Pfa4 regulates the ER exit of Chs3". J. Cell Biol. 174 (1): 19–25. doi:10.1083/jcb.200602049. PMC 2064155. PMID 16818716. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2064155.
- Ohno Y, Kihara A, Sano T, Igarashi Y (April 2006). "Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins". Biochim. Biophys. Acta 1761 (4): 474–83. doi:10.1016/j.bbalip.2006.03.010. PMID 16647879.
- Mitchell DA, Vasudevan A, Linder ME, Deschenes RJ (June 2006). "Protein palmitoylation by a family of DHHC protein S-acyltransferases". J. Lipid Res. 47 (6): 1118–27. doi:10.1194/jlr.R600007-JLR200. PMID 16582420.
- Hou H, Subramanian K, LaGrassa TJ, et al. (November 2005). "The DHHC protein Pfa3 affects vacuole-associated palmitoylation of the fusion factor Vac8". Proc. Natl. Acad. Sci. U.S.A. 102 (48): 17366–71. doi:10.1073/pnas.0508885102. PMC 1297695. PMID 16301533. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1297695.
- Smotrys JE, Schoenfish MJ, Stutz MA, Linder ME (September 2005). "The vacuolar DHHC-CRD protein Pfa3p is a protein acyltransferase for Vac8p". J. Cell Biol. 170 (7): 1091–9. doi:10.1083/jcb.200507048. PMC 2171546. PMID 16186255. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2171546.
- Gleason EJ, Lindsey WC, Kroft TL, Singson AW, L'hernault SW (January 2006). "spe-10 encodes a DHHC-CRD zinc-finger membrane protein required for endoplasmic reticulum/Golgi membrane morphogenesis during Caenorhabditis elegans spermatogenesis". Genetics 172 (1): 145–58. doi:10.1534/genetics.105.047340. PMC 1456142. PMID 16143610. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1456142.
- Seydel KB, Gaur D, Aravind L, Subramanian G, Miller LH (August 2005). "Plasmodium falciparum: characterization of a late asexual stage golgi protein containing both ankyrin and DHHC domains". Exp. Parasitol. 110 (4): 389–93. doi:10.1016/j.exppara.2005.03.030. PMID 15882865.
- Saitoh F, Tian QB, Okano A, Sakagami H, Kondo H, Suzuki T (July 2004). "NIDD, a novel DHHC-containing protein, targets neuronal nitric-oxide synthase (nNOS) to the synaptic membrane through a PDZ-dependent interaction and regulates nNOS activity". J. Biol. Chem. 279 (28): 29461–8. doi:10.1074/jbc.M401471200. PMID 15105416.
- Nagaya M, Inohaya K, Imai Y, Kudo A (December 2002). "Expression of zisp, a DHHC zinc finger gene, in somites and lens during zebrafish embryogenesis". Gene Expr. Patterns 2 (3-4): 355–8. doi:10.1016/S1567-133X(02)00021-2. PMID 12617825.
- Uemura T, Mori H, Mishina M (August 2002). "Isolation and characterization of Golgi apparatus-specific GODZ with the DHHC zinc finger domain". Biochem. Biophys. Res. Commun. 296 (2): 492–6. doi:10.1016/S0006-291X(02)00900-2. PMID 12163046.
- Li B, Cong F, Tan CP, Wang SX, Goff SP (August 2002). "Aph2, a protein with a zf-DHHC motif, interacts with c-Abl and has pro-apoptotic activity". J. Biol. Chem. 277 (32): 28870–6. doi:10.1074/jbc.M202388200. PMID 12021275.
Categories:- Protein domains
Wikimedia Foundation. 2010.
