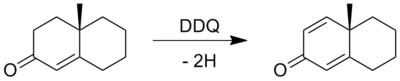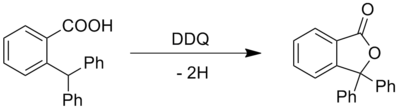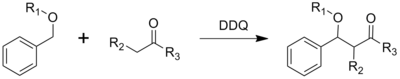- 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
-
- "DDQ" redirects here. DDQ is also the former callsign of a TV station in Toowoomba, Australia.
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone[1]  Other names
Other names- 2,3-Dichloro-5,6-dicyano-p-benzoquinone
- 4,5-Dichloro-3,6-dioxo-1,4-cyclohexadiene-1,2-dicarbonitrile
- Dichlorodicyanobenzoquinone
Identifiers Abbreviations DDQ CAS number 84-58-2 
PubChem 6775 ChemSpider 6517 
EC number 201-542-2 RTECS number GU4825000 Jmol-3D images Image 1 - ClC=1C(=O)C(\C#N)=C(\C#N)C(=O)C=1Cl
Properties Molecular formula C8Cl2N2O2 Molar mass 227 g mol−1 Appearance yellow to orange powder Density 1.7g/cm3 Melting point 210-215 °C (dec.)
Boiling point 301.8°C @ 760mmHg
Solubility in water reacts Hazards R-phrases R25 R29 S-phrases S22 S24/25 S37 S45 Flash point 136.3°C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
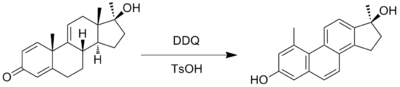
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C8Cl2N2O2. This oxidant is useful for the dehydrogenation of alcohols[2], phenols[3] and steroid ketones[4] in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.
Contents
Preparation
Synthesis of DDQ involves in cyanation and chlorination of benzoquinone. Thiele and Günther first reported a 6-step preparation in 1906[5]. A single-step chlorination from 2,3-dicyanohydroquinone was reported by Walker and Waugh in 1965.[2]
Stability
DDQ can react with water and give off hydrogen cyanide (HCN), which is highly toxic. Storage should be in dry area. Low temperature and weak acid environment can increase the stability of DDQ.
Uses
It is reagent used in organic chemistry as mild oxidizing agent and a radical receptor.
Reactions
1.Dehydrogenation
2.Aromatization
3.Oxidative Coupling
References
- ^ 2,3-Dichloro-5,6-dicyano-p-benzoquinone at Sigma-Aldrich
- ^ a b Braude. E.A,, Linstead, R. P., and Wooldridge, K. R. H. (Aug 1956). "Hydrogen Transfer. 9. The selective dehydrogenation of unsaturated alcohols by high-potential quinones". Journal of the American Chemical Society: 3070–3074. doi:10.1039/JR9560003070.
- ^ Becker. H.D, (1965). "Quinone Dehydrogenation .I. Oxidation Of Monohydric Phenols". Journal of Organic Chemistry 30 (4): 982. doi:10.1021/jo01015a006.
- ^ A.B. Turner, H.J Ringold (1967). "Applications of high-potential quinones. Part I. The mechanism of dehydrogenation of steroidal ketones by 2,3-dichloro-5,6-dicyanobenzoquinone". Journal of the Chemical Society: 1720–1730. doi:10.1039/J39670001720.
- ^ Thiele, J., and Gunther (1906). Ann.
- ^ Brown.W, Turner. AB; Turner, A. B. (1971). "Application of High-potential Quinones. 7. Synthesis of steroidal phenanthrenes by double methyl migration". J. Chem. Soc. C. (14): 2566–2572. doi:10.1039/J39710002566.
- ^ YH.Zhang, CJ. Li, and Wooldridge, K. R. H. (2006). "DDQ-Mediated Direct Cross-Dehydrogenative-Coupling (CDC) between Benzyl Ethers and Simple Ketones". Journal of the American Chemical Society 128 (13): 4242–4243. doi:10.1021/ja060050p.
External links
This article about a ketone is a stub. You can help Wikipedia by expanding it.