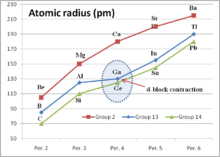
- d-block contraction
-
d-block contraction (sometimes called scandide contraction) is a term used in chemistry to describe the effect of having full d orbitals on the period 4 elements. The elements in question are the Ga, Ge, As, Se and Br. Their electronic configurations include completely filled d orbitals (d10). d-block contraction is best illustrated by comparing some properties of the group 13 elements to highlight the effect on gallium.
Element Atomic electron config. Sum 1st - 3d I.Ps kJ/mol M3+ electron config. M3+ radius (pm) Boron, B {He}2s22p1 6887.4 {He} Aluminium, Al {Ne}3s23p1 5139 {Ne} 53.5 Gallium, Ga {Ar}3d104s24p1 5521.1 {Ar}3d10 62 Indium, In {Kr}4d105s25p1 5083 {Kr} 4d10 80 Thallium, Tl {Xe}4f145d106s26p1 5438.4 {Xe}5d10 88.5 Gallium can be seen to be anomalous. The most obvious effect is that the sum of the first three ionization potentials of gallium is higher than that of aluminium, whereas the trend in the group would be for it to be lower. The second table below shows the trend in the sum of the first three ionization potentials for the elements B, Al, Sc, Y, La. Sc, Y, La are group 3 elements and have three valence electrons above a noble gas electron core. In contrast to the group 13 elements this sequence shows a smooth reduction.
Element Atomic electron config. Sum 1st - 3d I.Ps kJ/mol M3+ electron config. M3+ radius (pm) Boron, B {He}2s22p1 6887.4 {He} Aluminium, Al {Ne}3s23p1 5139 {Ne} 53.5 Scandium, Sc {Ar}3d14s2 4256.7 {Ar} 74.5 Yttrium, Y {Kr}4d15s2 3760 {Kr} 90 Lanthanum, La {Xe}5d16s2 3455.4 {Xe} 103.2 Other effects of the d-block contraction are that the Ga3+ ion is smaller than expected, being closer in size to Al3+. Care must be taken in interpreting the ionization potentials for indium and thallium, other effects e.g. the inert pair effect become increasingly important for the heavier members of the group.
The cause of the d-block contraction is the poor shielding of the nuclear charge by the electrons in the d orbitals. The outer valence electrons are more strongly attracted by the nucleus causing the observed increase in ionization potentials. The d-block contraction can be compared to the lanthanide contraction which is caused by inadequate shielding of nuclear charge by electrons occupying f orbitals.References
Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0080379419.
Categories:- Chemical bonding
Wikimedia Foundation. 2010.