- Cerium(III) oxide
-
Cerium(III) oxide 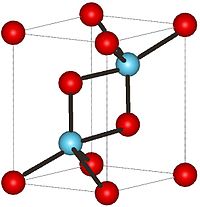 Cerium(III) oxideOther namesCerium sesquioxide
Cerium(III) oxideOther namesCerium sesquioxideIdentifiers CAS number 1345-13-7 
Properties Molecular formula Ce2O3 Molar mass 328.24 g/mol Appearance yellow/green pulver, Density 6.2 g/cm3 Melting point 2177 °C
Solubility in water insoluble Solubility in sulfuric acid soluble Solubility in hydrochloric acid insoluble Structure Crystal structure Hexagonal, hP5 Space group P-3m1, No. 164 Related compounds Related compounds CeO2  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cerium(III) oxide is an oxide of the rare earth metal cerium. It has chemical formula Ce2O3, and is gold-yellow in color.
Contents
Applications
Exhaust catalysts
Cerium oxide is used as a catalytic converter for the minimisation of CO emissions in the exhaust gases from motor vehicles.
When there is a shortage of oxygen, cerium(IV) oxide is reduced by carbon monoxide to cerium(III) oxide:
- 2 CeO2 + CO → Ce2O3 + CO2
When there is an oxygen surplus, the process is reversed and cerium(III) oxide is oxidized to cerium(IV) oxide:
- 2 Ce2O3 + O2 → 4 CeO2
Water splitting
The cerium(IV) oxide-cerium(III) oxide cycle or CeO2/Ce2O3 cycle is a two step thermochemical water splitting process based on cerium(IV) oxide and cerium(III) oxide for hydrogen production.[1]
Illumination
Cerium(III) oxide combined with tin(II) oxide (SnO) in ceramic form is used for illumination with UV light. It absorbs light with a wavelength of 320 nm and emits light with a wavelength of 412 nm.[2] This combination of cerium(III) oxide and tin(II) oxide is rare, and obtained only with difficulty on a laboratory scale.
Production
Cerium(III) oxide is produced by the reduction of cerium(IV) oxide with hydrogen at approximately 1,400 °C (2,550 °F) to make air stable cerium(III) oxide. Production at other temperatures results in pyrophoric cerium(III) oxide.[3]
References
- ^ Hydrogen production from solar thermochemical water splitting cycles
- ^ Spectral Studies of New Luminophors for Dental Porcelain
- ^ Georg Brauer: Handbuch der Präparativen Anorganischen Chemie Band II, Seite 1090, Ferdinand Enke Verlag Stuttgart 1978, ISBN 3-432-87813-3
External links
Cerium compounds
Wikimedia Foundation. 2010.
Look at other dictionaries:
Cerium(IV) oxide-cerium(III) oxide cycle — Simplified diagram of the Cerium(IV) oxide cerium(III) oxide cycle The cerium(IV) oxide cerium(III) oxide cycle or CeO2/Ce2O3 cycle is a two step thermochemical process based on cerium(IV) oxide and cerium(III) oxide for hydrogen production.[1] … Wikipedia
Cerium(IV) oxide — Cerium(IV) oxide … Wikipedia
Iron(III) oxide — Iron(III) oxide … Wikipedia
Cerium oxalate — IUPAC … Wikipedia
Cerium oxide — may refer to either of the following: Cerium(III) oxide, Ce2O3 Cerium(IV) oxide, CeO2 This disambiguation page lists articles associated with the same title. If an internal l … Wikipedia
Cerium — lanthanum ← cerium → praseodymium ↑ Ce ↓ Th … Wikipedia
Cerium uranium blue — is the name given to solid solutions of cerium(IV) oxide, CeO2, and uranium(IV) oxide, UO2, of variable composition from 0 to 100% uranium. Synthesis and properties Cerium uranium blue was first obtained by heating together cerium sulfate and… … Wikipedia
Cerium — Eigenschaften … Deutsch Wikipedia
Neodymium(III) chloride — Other names … Wikipedia
Terbium(III,IV) oxide — Chembox new Name = Terbium(III,IV) oxide ImageFile = Tetraterbium heptaoxide.jpg ImageName = Terbium(III,IV) oxide IUPACName = Tetraterbium heptaoxide OtherNames = Terbium(III,IV) oxide, Terbium peroxide Section1 = Chembox Identifiers CASNo =… … Wikipedia
