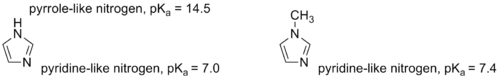- 1-Methylimidazole
-
1-Methylimidazole  1-MethylimidazoleOther namesN-Methylimidazole
1-MethylimidazoleOther namesN-MethylimidazoleIdentifiers CAS number 616-47-7 
ChemSpider 1348 
ChEBI CHEBI:113454 
ChEMBL CHEMBL543 
Jmol-3D images Image 1
Image 2- n1ccn(c1)C
Cn1ccnc1
Properties Molecular formula C4H6N2 Molar mass 82.10 g/mol Density 1.03 g/cm³ Melting point -60 °C
Boiling point 198 °C
Hazards MSDS Oxford MSDS EU classification Harmful (Xn); Corrosive (C)  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1-Methylimidazole or N-Methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. Its N-methylation removes the possibility of tautomerization, which occurs in imidazole and many imidazole derivatives. 1-Methylimidazole maintains the pyridine-like nitrogen of imidazole, only with a slightly higher pKa.[1] The methylation also provides a significantly lower melting point, which makes 1-methylimidazole a useful solvent.
1-Methylimidazole has found use as a solvent, a base, a catalyst, a mimic for purine nucleoside bases and histidine and histamine, and as an ionic liquid precursor.
Contents
Synthesis
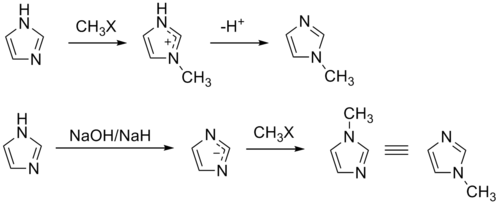
1-Methylimidazole is synthesized on an industrial scale by the Radziszewski reaction from glyoxal, formaldehyde, and a mixture of ammonia and methylamine.[2][3]
The compound can also be synthesized on a laboratory scale by methylation of imidazole at the pyridine-like nitrogen and subsequent deprotonation.[4] Similarly, 1-methylimidazole may be synthesized by first deprotonating imidazole to form a sodium salt followed by methylation.[5][6]
Biomolecule analog
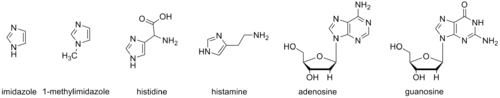
The imidazole backbone is an essential functional unit in biology. The amino acid histidine, the signaling molecule histamine, and the purine nucleobases all contain an imidazole ring.[2]
1-Methylimidazole and its derivatives have been used to mimic aspects of these biomolecules. These mimics can be useful in studies to elucidate biological mechanisms and as portions of synthetic bioactive molecules.
1-Methylimidazole is also the precursor for the synthesis of the methylimidazole monomer of pyrrole-imidazole polyamides. These polymers can selectively bind specific sequences of double-stranded DNA by intercalating in a sequence dependent manner.[7]
Ionic liquid precursor
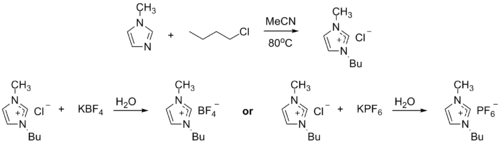
1-Methylimidazole alkylates to form dialkyl imidazolium salts. Depending on the alkylating agent and the counteranion, various ionic liquids result, e.g. 1-butyl-3-methylimidazolium hexafluorophosphate ("BMIMPF6"):[8][9]
BASF has used 1-methylimidazole as a means to remove acid during their industrial-scale production of diethoxyphenylphosphine. In this biphasic acid scavenging using ionic liquids (BASIL) process, 1-methylimidazole reacts with HCl to produce 1-methylimidazolium chloride, a salt that is easily separated and deprotonated to regenerate 1-methylimidazole.[8]
- 2 MeC3N2H3 + C6H5PCl2 + 2 C2H5OH → 2 [MeC3N2H4]Cl + C6H5P(OC2H5)2
References
- ^ Albert, A., Heterocyclic Chemistry, 2nd ed.; 1968 Athlone Press, ISBN 048511092X
- ^ a b Ebel, K., Koehler, H., Gamer, A. O., & Jäckh, R. “Imidazole and Derivatives.” In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, doi:10.1002/14356007.a13_661
- ^ Bronislas Radziszewski (1882). "Ueber die Constitution des Lophins und verwandter Verbindungen". Berichte der deutschen chemischen Gesellschaft 15 (2): 1493–1496. doi:10.1002/cber.18820150207.
- ^ Gilchrist, T. L., Heterocyclic Chemistry, 2nd ed.; 1992 Longman Scientific & Technical, ISBN 0-582-06420-1
- ^ Grimmett, M. R., Imidazole and Benzimidazole Synthesis; 1997 Academic Press, ISBN 0-12-303190-7
- ^ Gupta, R. R., Kumar, M., Gupta, V., Heterocyclic Chemistry II: Five Membered Heterocycles; 1999 Springer, ISBN 3-540-65252-3
- ^ Baird, E.E. & Dervan, P.B. J. Am. Chem. Soc. 118 (26), 6141-6146, 1996.
- ^ a b Meindersma, W., Maase, M., and De Haan, A. B. “Ionic Liquids.” In Ullmann’s Encyclopedia of Industrial Chemistry; 2007 Wiley-VCH, doi:10.1002/14356007.l14_l01
- ^ Dupont, J., Consorti, C., Suarez, P., de Souza, R. (2004), "Preparation of 1-Butyl-3-methyl imidazolium-based Room Temperature Ionic Liquids", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v79p0236; Coll. Vol. 10: 184
Categories:- Imidazoles
- n1ccn(c1)C
Wikimedia Foundation. 2010.