- Contact angle
-
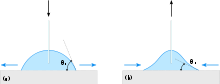
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat horizontal solid surface. The shape of the droplet is determined by the Young-Laplace equation. The contact angle plays the role of a boundary condition. Contact angle is measured using a contact angle goniometer. The contact angle is not limited to a liquid/vapour interface; it is equally applicable to the interface of two liquids.
Contents
Typical contact angles
Consider a liquid drop on a solid surface. If the liquid is very strongly attracted to the solid surface (for example water on a strongly hydrophilic solid) the droplet will completely spread out on the solid surface and the contact angle will be close to 0°. Less strongly hydrophilic solids will have a contact angle up to 90°. On many highly hydrophilic surfaces, water droplets will exhibit contact angles of 0° to 30°. If the solid surface is hydrophobic, the contact angle will be larger than 90°. On highly hydrophobic surfaces the surfaces have water contact angles as high as ~120° on low energy materials e.g. fluorinated surfaces. However, some materials with highly rough surfaces may have a water contact angle greater than 150°. These are called superhydrophobic surfaces. Sometimes the contact angle is measured through the gas instead of through the liquid, which reverses 0 and 180 in the above explanation.[1][2]
Thermodynamics
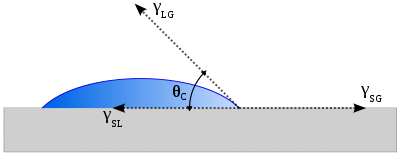
The theoretical description of contact arises from the consideration of a thermodynamic equilibrium between the three phases: the liquid phase of the droplet (L), the solid phase of the substrate (S), and the gas/vapor phase of the ambient (G) (which will be a mixture of ambient atmosphere and an equilibrium concentration of the liquid vapor). The gaseous phase could also be another (immiscible) liquid phase. At equilibrium, the chemical potential in the three phases should be equal. It is convenient to frame the discussion in terms of the interfacial energies. We denote the solid–vapor interfacial energy (see surface energy) as γSG, the solid–liquid interfacial energy as γSL and the liquid–vapor energy (i.e. the surface tension) as simply γ, we can write an equation that must be satisfied in equilibrium (known as the Young Equation):
where θC is the equilibrium contact angle. The Young equation assumes a perfectly flat surface, and in many cases surface roughness and impurities cause a deviation in the equilibrium contact angle from the contact angle predicted by Young's equation. Even in a perfectly smooth surface a drop will assume a wide spectrum of contact angles between the highest (advancing) contact angle, θA, and the lowest (receding) contact angle, θR. The equilibrium contact angle (θC) can be calculated from θA and θR as was shown theoretically by Tadmor [3] and confirmed experimentally by Chibowski [4]:
Where,
and,
The contact angle can also be used to determine an interfacial energy (if other interfacial energies are known). This equation can be rewritten as the Young-Dupre equation:
where ΔWSLV is the adhesion energy per unit area of the solid and liquid surfaces when in the medium V.
Measuring methods
- The static sessile drop method
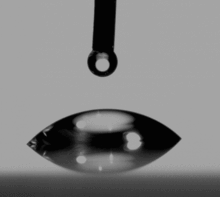
- The sessile drop method is measured by a contact angle goniometer using an optical subsystem to capture the profile of a pure liquid on a solid substrate. The angle formed between the liquid/solid interface and the liquid/vapor interface is the contact angle. Older systems used a microscope optical system with a back light. Current-generation systems employ high resolution cameras and software to capture and analyze the contact angle.
- The dynamic sessile drop method
- The dynamic sessile drop is similar to the static sessile drop but requires the drop to be modified. A common type of dynamic sessile drop study determines the largest contact angle possible without increasing its solid/liquid interfacial area by adding volume dynamically. This maximum angle is the advancing angle. Volume is removed to produce the smallest possible angle, the receding angle. The difference between the advancing and receding angle is the contact angle hysteresis.
- Dynamic Wilhelmy method
- A method for calculating average advancing and receding contact angles on solids of uniform geometry. Both sides of the solid must have the same properties. Wetting force on the solid is measured as the solid is immersed in or withdrawn from a liquid of known surface tension.
- Single-fiber Wilhelmy method
- Dynamic Wilhelmy method applied to single fibers to measure advancing and receding contact angles.
- Powder contact angle method
- Enables measurement of average contact angle and sorption speed for powders and other porous materials. Change of weight as a function of time is measured.
See also
References
- ^ Renate Förch, Holger Schönherr, A. Tobias A. Jenkins (2009). Surface design: applications in bioscience and nanotechnology. Wiley-VCH. p. 471. ISBN 3527407898. http://books.google.com/?id=cvn5l-QytVIC&pg=PA471.
- ^ P.G. de Gennes (1985). "Wetting: statics and dynamics". Reviews of Modern Physics 57 (3): 827–863. Bibcode 1985RvMP...57..827D. doi:10.1103/RevModPhys.57.827.
- ^ Tadmor, Rafael (2004). "Line Energy and the Relation between Advancing, Receding, and Young Contact Angles". Langmuir 20 (18): 7659. doi:10.1021/la049410h. PMID 15323516.
- ^ Chibowski, Emil (2008). "Surface free energy of sulfur—Revisited I. Yellow and orange samples solidified against glass surface". Journal of Colloid and Interface Science 319: 505. doi:10.1016/j.jcis.2007.10.059.
Further reading
- Jacob Israelachvili, Intermolecular and Surface Forces, Academic Press (1985–2004)
- D.W. Van Krevelen, Properties of Polymers, 2nd revised edition, Elsevier Scientific Publishing Company, Amsterdam-Oxford-New York (1976)
Categories:- Condensed matter physics
- Angle
- Fluid mechanics
- Surface chemistry
Wikimedia Foundation. 2010.






![r_\mathrm{A}=\sqrt[3]{\frac{\sin^3{\theta_\mathrm{A}}}{2-3\cos{\theta_\mathrm{A}}+\cos^3{\theta_\mathrm{A}}}}](e/ddee34f85df18f190883f956317e27c0.png)
![r_\mathrm{R}=\sqrt[3]{\frac{\sin^3{\theta_\mathrm{R}}}{2-3\cos{\theta_\mathrm{R}}+\cos^3{\theta_\mathrm{R}}}}](7/c879d3187825546a138c0f4c4db4a771.png)