- 4,4'-Dichlorodiphenyl sulfone
-
4,4'-Dichlorodiphenyl sulfone 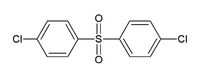 Other namesbis(4-chlorophenyl) sulfone; bis(p-chlorophenyl) sulfone; 4,4'-Dichlorodiphenylsulfone; DCDPS
Other namesbis(4-chlorophenyl) sulfone; bis(p-chlorophenyl) sulfone; 4,4'-Dichlorodiphenylsulfone; DCDPSIdentifiers CAS number 80-07-9 ChemSpider 6373 
Jmol-3D images Image 1
Image 2- O=S(=O)(c1ccc(Cl)cc1)c2ccc(Cl)cc2
O=S(=O)(c1ccc(Cl)cc1)c2ccc(Cl)cc2
Properties Molecular formula C12H8Cl2O2S Molar mass 287.16 g/mol Appearance White solid Melting point 143 °C, 416 K, 289 °F
Boiling point 250 °C, 523 K, 482 °F
Solubility in water Insoluble Hazards MSDS External MSDS  sulfone (verify) (what is:
sulfone (verify) (what is:  /
/ ?)
?)
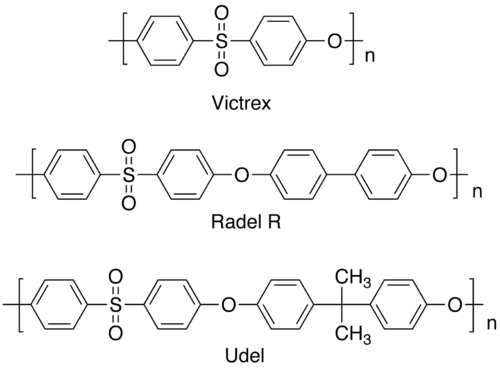
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 4,4'-Dichlorodiphenyl sulfone, abbreviated as DCDPS, is an organic sulfone with the formula (ClC6H4)2SO2. It is most commonly used as a precursor to polymers that are rigid and temperature resistant such as Victrex or Udel. The compound is readily available through chemical suppliers in its powdered form.
Synthesis
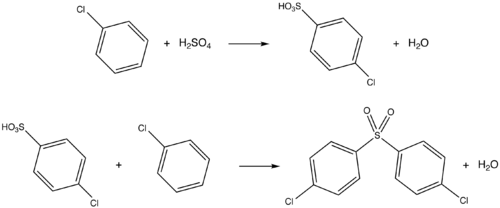
DCDPS can be synthesized by treating chlorobenzene with sulfuric acid at a temperature of 200-250 °C. The reaction can be done in the presence of boric acid or trifluoromethanesulfonic acid, which increase the yield DCDPS by reducing the formation of the 2,4 and 3,4 isomers. The reaction goes to completion in approximately 10 hours and produces a high yield of 4,4'-dichlorodiphenyl sulfone.[citation needed]
Uses
DCDPS is used as a starting material in the polymerization of compounds such as Udel, Victrex, and Radel R. The polymerization occurs through a nucleophilic substitution reaction of DCDPS with difunctional nucleophiles. With bisphenol A in dimethyl sulfoxide, DCDPS forms a material called Udel. Udel is a high performance amorphous sulfone polymer that can molded into a variety of different shapes. It is both rigid and temperature resistant, and has applications in everything from plumbing pipes, to printer cartridges, to automobile fuses. DCDPS also reacts with bisphenol S to form Victrex. Like Udel, Victrex is a rigid and thermally resistant material with numerous applications.
The general polymerization reaction:
Some of the products include:
References
- “Polymers, High-Temperature,” David Parker, Jan Bussink, Hendrik T. van de Grampel, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2002, 10.1002/14356007.a21_449
- “Chlorination of Aromatic Compounds with Metal Chlorides,” Kovacic, Peter and Brace, Neal O. J. Am. Chem. Soc., 76, 21, 5491 – 5494, 10.1021/ja01650a069
- “Synthesis of Aryl Sulfones,” Graybill, Bruce M. J. Org. Chem., 32, 9, 2931 – 2933, 1967, 10.1021/jo01284a075
- "Udel Polysulfone Design Guide," Solvay Advanced Polymers LCC, pp 7–10, Alpharetta, GA.
Categories:- Sulfones
- O=S(=O)(c1ccc(Cl)cc1)c2ccc(Cl)cc2
Wikimedia Foundation. 2010.