- Carbon diselenide
-
Carbon diselenide 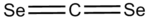

 Other namesCarbon Selenide, Diselenoxomethane, Methanediselone
Other namesCarbon Selenide, Diselenoxomethane, MethanediseloneIdentifiers CAS number 506-80-9 Properties Molecular formula CSe2 Molar mass 169.93 g/mol Appearance yellow liquid Density 2.69 g/cm3 Melting point −43.7 °C
Boiling point 125.5 °C
Solubility in water insoluble in water  diselenide (verify) (what is:
diselenide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Carbon diselenide is an inorganic compound with the chemical formula CSe2. It is a yellow-orange oily liquid with pungent odor. It is an analogue of carbon disulfide (CS2). This light-sensitive compound is insoluble in water and soluble in organic solvents.
Synthesis, structure and reactions
Carbon diselenide is a linear molecule with D∞h symmetry. It is produced by reacting selenium powder with dichloromethane vapor near 550 °C.[1]
- 2 Se + CH2Cl2 → CSe2 + 2 HCl
It was first reported by Grimm and Metzger, who prepared it by treating hydrogen selenide with carbon tetrachloride in a hot tube.[2]
Like carbon disulfides, carbon diselenide polymerizes under high pressure. The structure of the polymer is thought to be a head-to-head structure with a backbone in the form of –[Se–C(=Se)–C(=Se)–Se]–.[3] The polymer is a semiconductor with a room-temperature conductivity of 50 S/cm.
In addition, carbon diselenide is a precursor to tetraselenafulvalenes,[4] the selenium analogue of tetrathiafulvalene, which can be further used to synthesize organic conductors and organic superconductors.
Carbon diselenide reacts with secondary amines to give dialkydiselenocarbamates:[1]
- 2 Et2NH + CSe2 → (Et2NH2+)(Et2NCSe2−)
Safety
Carbon diselenide has high vapor pressure. It is toxic and presents an inhalation hazard. It may be dangerous due to its easy membrane transport. It decomposes slowly in storage (about 1% per month at –30 °C). When obtained commercially, its cost is high.[5]
Mixed with air, CSe2 releases an extremely offensive odor.[6] Its smell forced an evacuation of a nearby village when it was first synthetized in 1936. The stench can be neutralized by oxidizing with bleach.[7] Because of the odor, synthetic pathways have been developed to avoid its use.[8]
References
- ^ a b W. Pan and J. P. Fackler, Jr. (1982). "Diselenocarbamates from carbon diselenide". Inorganic Syntheses. Inorganic Syntheses 21: 6. doi:10.1002/9780470132524.ch2. ISBN 9780470132524.
- ^ H. G. Grimm, H. Metzger (1936). "Über Darstellung und Eigenschaften des Selenkohlenstoffs". Berichte der deutschen chemischen Gesellschaft (A and B Series) 69 (6): 1356–1364. doi:10.1002/cber.19360690626.
- ^ C. E. Carraher, Jr. and C.U. Pittman, Jr. (2005). "Poly(Carbon Disulfide), Poly(Carbon Diselenide), and Polythiocyanogen". Inorganic Polymers 21. doi:10.1002/14356007.a14_241. ISBN 3527306730.
- ^ Edward M. Engler, Vishnu V. Patel (1974). "Structure control in organic metals. Synthesis of tetraselenofulvalene and its charge transfer salt with tetracyano-p-quinodimethane". Journal of American Chemical Society 96 (23): 7376–7378. doi:10.1021/ja00810a042.
- ^ "Carbon Diselenide CSe2". Cse2.com. http://www.cse2.com/. Retrieved 2010-02-27.
- ^ ""carbon diselenide has by far the worst odor this author has experienced in his lifetime of working with selenium compounds"" Wolfgang H.H. Gunther: Organic Selenium Compounds: Their Chemistry and Biology
- ^ Derek. "Things I Won't Work With: Carbon Diselenide. In the Pipeline:". Pipeline.corante.com. http://pipeline.corante.com/archives/2005/03/03/things_i_wont_work_with_carbon_diselenide.php. Retrieved 2010-02-27.
- ^ "Process for producing chalcogen containing compounds - Patent 4462938". Freepatentsonline.com. http://www.freepatentsonline.com/4462938.html. Retrieved 2010-02-27.
Categories:- Selenides
- Inorganic carbon compounds
- Foul-smelling chemicals
Wikimedia Foundation. 2010.
