- OSBP
-
Oxysterol binding protein Identifiers Symbols OSBP; OSBP1 External IDs OMIM: 167040 MGI: 97447 HomoloGene: 97668 GeneCards: OSBP Gene Gene Ontology Molecular function • oxysterol binding
• lipid bindingCellular component • Golgi membrane
• cytoplasm
• Golgi apparatus
• membraneBiological process • transport
• lipid transportSources: Amigo / QuickGO RNA expression pattern 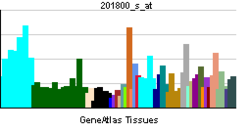
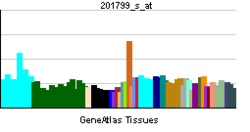
More reference expression data Orthologs Species Human Mouse Entrez 5007 76303 Ensembl ENSG00000110048 ENSMUSG00000024687 UniProt P22059 n/a RefSeq (mRNA) NM_002556 NM_001033174.1 RefSeq (protein) NP_002547 NP_001028346.1 Location (UCSC) Chr 11:
59.34 – 59.38 MbChr 19:
12.04 – 12.07 MbPubMed search [1] [2] Oxysterol-binding protein 1 is a protein that in humans is encoded by the OSBP gene.[1]
Oxysterol binding protein is an intracellular protein that is believed to transport sterols from lysosomes to the nucleus where the sterol down-regulates the genes for the LDL receptor, HMG-CoA reductase, and HMG synthetase[1]
Interactions
OSBP has been shown to interact with VAPA.[2]
References
- ^ a b "Entrez Gene: OSBP oxysterol binding protein". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=5007.
- ^ Wyles, Jessica P; McMaster Christopher R, Ridgway Neale D (Aug. 2002). "Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum". J. Biol. Chem. (United States) 277 (33): 29908–18. doi:10.1074/jbc.M201191200. ISSN 0021-9258. PMID 12023275.
Further reading
- Ridgway ND, Dawson PA, Ho YK et al. (1992). "Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding". J. Cell Biol. 116 (2): 307–19. doi:10.1083/jcb.116.2.307. PMC 2289278. PMID 1730758. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2289278.
- Levanon D, Hsieh CL, Francke U et al. (1990). "cDNA cloning of human oxysterol-binding protein and localization of the gene to human chromosome 11 and mouse chromosome 19". Genomics 7 (1): 65–74. doi:10.1016/0888-7543(90)90519-Z. PMID 1970801.
- Laitinen S, Olkkonen VM, Ehnholm C, Ikonen E (2000). "Family of human oxysterol binding protein (OSBP) homologues. A novel member implicated in brain sterol metabolism". J. Lipid Res. 40 (12): 2204–11. PMID 10588946.
- Moreira EF, Jaworski C, Li A, Rodriguez IR (2001). "Molecular and biochemical characterization of a novel oxysterol-binding protein (OSBP2) highly expressed in retina". J. Biol. Chem. 276 (21): 18570–8. doi:10.1074/jbc.M011259200. PMID 11278871.
- Jaworski CJ, Moreira E, Li A et al. (2002). "A family of 12 human genes containing oxysterol-binding domains". Genomics 78 (3): 185–96. doi:10.1006/geno.2001.6663. PMID 11735225.
- Wyles JP, McMaster CR, Ridgway ND (2002). "Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum". J. Biol. Chem. 277 (33): 29908–18. doi:10.1074/jbc.M201191200. PMID 12023275.
- Strausberg RL, Feingold EA, Grouse LH et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Beausoleil SA, Jedrychowski M, Schwartz D et al. (2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proc. Natl. Acad. Sci. U.S.A. 101 (33): 12130–5. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=514446.
- Gerhard DS, Wagner L, Feingold EA et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
- Wang PY, Weng J, Anderson RG (2005). "OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation". Science 307 (5714): 1472–6. doi:10.1126/science.1107710. PMID 15746430.
- Perry RJ, Ridgway ND (2006). "Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein". Mol. Biol. Cell 17 (6): 2604–16. doi:10.1091/mbc.E06-01-0060. PMC 1474796. PMID 16571669. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1474796.
- Olsen JV, Blagoev B, Gnad F et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
Categories:- Human proteins
- Chromosome 11 gene stubs
Wikimedia Foundation. 2010.
