- DOK2
-
Docking protein 2, 56kDa 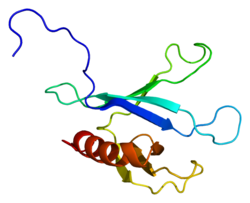
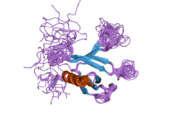
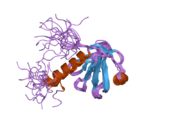
PDB rendering based on 2d9w.Available structures PDB 2D9W, 2DLW Identifiers Symbols DOK2; p56DOK; p56dok-2 External IDs OMIM: 604997 MGI: 1332623 HomoloGene: 2957 GeneCards: DOK2 Gene Gene Ontology Molecular function • receptor signaling protein activity
• transmembrane receptor protein tyrosine kinase adaptor activity
• insulin receptor binding
• protein binding
• identical protein bindingCellular component • cytosol Biological process • signal transduction
• cell surface receptor linked signaling pathway
• Ras protein signal transduction
• blood coagulation
• leukocyte migrationSources: Amigo / QuickGO RNA expression pattern 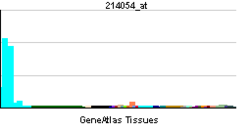
More reference expression data Orthologs Species Human Mouse Entrez 9046 13449 Ensembl ENSG00000147443 ENSMUSG00000022102 UniProt O60496 Q3TX09 RefSeq (mRNA) NM_003974 NM_010071.2 RefSeq (protein) NP_003965 NP_034201.1 Location (UCSC) Chr 8:
21.77 – 21.77 MbChr 14:
71.17 – 71.18 MbPubMed search [1] [2] Docking protein 2 is a protein that in humans is encoded by the DOK2 gene.[1][2][3]
The protein encoded by this gene is constitutively tyrosine phosphorylated in hematopoietic progenitors isolated from chronic myelogenous leukemia (CML) patients in the chronic phase. It may be a critical substrate for p210(bcr/abl), a chimeric protein whose presence is associated with CML. This encoded protein binds p120 (RasGAP) from CML cells.[3]
Interactions
DOK2 has been shown to interact with INPP5D[4] and TEK tyrosine kinase.[5][6]
References
- ^ Di Cristofano A, Carpino N, Dunant N, Friedland G, Kobayashi R, Strife A, Wisniewski D, Clarkson B, Pandolfi PP, Resh MD (Mar 1998). "Molecular cloning and characterization of p56dok-2 defines a new family of RasGAP-binding proteins". J Biol Chem 273 (9): 4827–30. doi:10.1074/jbc.273.9.4827. PMID 9478921.
- ^ Garcia A, Prabhakar S, Hughan S, Anderson TW, Brock CJ, Pearce AC, Dwek RA, Watson SP, Hebestreit HF, Zitzmann N (Mar 2004). "Differential proteome analysis of TRAP-activated platelets: involvement of DOK-2 and phosphorylation of RGS proteins". Blood 103 (6): 2088–95. doi:10.1182/blood-2003-07-2392. PMID 14645010.
- ^ a b "Entrez Gene: DOK2 docking protein 2, 56kDa". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=9046.
- ^ Dunant, N M; Wisniewski D, Strife A, Clarkson B, Resh M D (May. 2000). "The phosphatidylinositol polyphosphate 5-phosphatase SHIP1 associates with the dok1 phosphoprotein in bcr-Abl transformed cells". Cell. Signal. (ENGLAND) 12 (5): 317–26. doi:10.1016/S0898-6568(00)00073-5. ISSN 0898-6568. PMID 10822173.
- ^ Jones, N; Dumont D J (Sep. 1998). "The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R". Oncogene (ENGLAND) 17 (9): 1097–108. doi:10.1038/sj.onc.1202115. ISSN 0950-9232. PMID 9764820.
- ^ Master, Z; Jones N, Tran J, Jones J, Kerbel R S, Dumont D J (Nov. 2001). "Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak". EMBO J. (England) 20 (21): 5919–28. doi:10.1093/emboj/20.21.5919. ISSN 0261-4189. PMC 125712. PMID 11689432. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=125712.
Further reading
- Jiang H, Harris MB, Rothman P (2000). "IL-4/IL-13 signaling beyond JAK/STAT.". J. Allergy Clin. Immunol. 105 (6 Pt 1): 1063–70. doi:10.1067/mai.2000.107604. PMID 10856136.
- Jones N, Dumont DJ (1998). "The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R.". Oncogene 17 (9): 1097–108. doi:10.1038/sj.onc.1202115. PMID 9764820.
- Jones N, Dumont DJ (2000). "Recruitment of Dok-R to the EGF receptor through its PTB domain is required for attenuation of Erk MAP kinase activation.". Curr. Biol. 9 (18): 1057–60. doi:10.1016/S0960-9822(99)80458-8. PMID 10508618.
- Némorin JG, Duplay P (2000). "Evidence that Llck-mediated phosphorylation of p56dok and p62dok may play a role in CD2 signaling.". J. Biol. Chem. 275 (19): 14590–7. doi:10.1074/jbc.275.19.14590. PMID 10799545.
- Dunant NM, Wisniewski D, Strife A, et al. (2000). "The phosphatidylinositol polyphosphate 5-phosphatase SHIP1 associates with the dok1 phosphoprotein in bcr-Abl transformed cells.". Cell. Signal. 12 (5): 317–26. doi:10.1016/S0898-6568(00)00073-5. PMID 10822173.
- Némorin JG, Laporte P, Bérubé G, Duplay P (2001). "p62dok negatively regulates CD2 signaling in Jurkat cells.". J. Immunol. 166 (7): 4408–15. PMID 11254695.
- Grimm J, Sachs M, Britsch S, et al. (2001). "Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation.". J. Cell Biol. 154 (2): 345–54. doi:10.1083/jcb.200102032. PMC 2150770. PMID 11470823. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2150770.
- Master Z, Jones N, Tran J, et al. (2001). "Dok-R plays a pivotal role in angiopoietin-1-dependent cell migration through recruitment and activation of Pak.". EMBO J. 20 (21): 5919–28. doi:10.1093/emboj/20.21.5919. PMC 125712. PMID 11689432. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=125712.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences.". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Salomon AR, Ficarro SB, Brill LM, et al. (2003). "Profiling of tyrosine phosphorylation pathways in human cells using mass spectrometry.". Proc. Natl. Acad. Sci. U.S.A. 100 (2): 443–8. doi:10.1073/pnas.2436191100. PMC 141014. PMID 12522270. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=141014.
- Jones N, Chen SH, Sturk C, et al. (2003). "A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function.". Mol. Cell. Biol. 23 (8): 2658–68. doi:10.1128/MCB.23.8.2658-2668.2003. PMC 152553. PMID 12665569. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=152553.
- Master Z, Tran J, Bishnoi A, et al. (2003). "Dok-R binds c-Abl and regulates Abl kinase activity and mediates cytoskeletal reorganization.". J. Biol. Chem. 278 (32): 30170–9. doi:10.1074/jbc.M301339200. PMID 12777393.
- Gérard A, Favre C, Garçon F, et al. (2004). "Functional interaction of RasGAP-binding proteins Dok-1 and Dok-2 with the Tec protein tyrosine kinase.". Oncogene 23 (8): 1594–8. doi:10.1038/sj.onc.1207283. PMID 14647425.
- Brill LM, Salomon AR, Ficarro SB, et al. (2004). "Robust phosphoproteomic profiling of tyrosine phosphorylation sites from human T cells using immobilized metal affinity chromatography and tandem mass spectrometry.". Anal. Chem. 76 (10): 2763–72. doi:10.1021/ac035352d. PMID 15144186.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC).". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=528928.
- Rush J, Moritz A, Lee KA, et al. (2005). "Immunoaffinity profiling of tyrosine phosphorylation in cancer cells.". Nat. Biotechnol. 23 (1): 94–101. doi:10.1038/nbt1046. PMID 15592455.
- Van Slyke P, Coll ML, Master Z, et al. (2005). "Dok-R mediates attenuation of epidermal growth factor-dependent mitogen-activated protein kinase and Akt activation through processive recruitment of c-Src and Csk.". Mol. Cell. Biol. 25 (9): 3831–41. doi:10.1128/MCB.25.9.3831-3841.2005. PMC 1084282. PMID 15831486. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1084282.
PDB gallery Categories:- Human proteins
- Chromosome 8 gene stubs
Wikimedia Foundation. 2010.