- Cystatin A
-
Cystatin A (stefin A) 
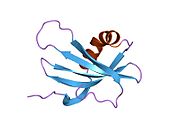
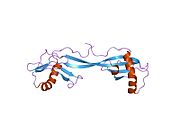
PDB rendering based on 1cyu.Available structures PDB 1CYU, 1CYV, 1DVC, 1DVD, 1GD3, 1GD4, 1N9J, 1NB3, 1NB5, 3K9M, 3KFQ Identifiers Symbols CSTA; STF1; STFA External IDs OMIM: 184600 MGI: 3524930 HomoloGene: 3819 GeneCards: CSTA Gene Gene Ontology Molecular function • protease binding
• cysteine-type endopeptidase inhibitor activity
• structural molecule activity
• protein binding, bridgingCellular component • cornified envelope
• intracellular
• nucleus
• cytoplasmBiological process • negative regulation of peptidase activity
• peptide cross-linking
• keratinocyte differentiationSources: Amigo / QuickGO RNA expression pattern 
More reference expression data Orthologs Species Human Mouse Entrez 1475 76770 Ensembl ENSG00000121552 ENSMUSG00000051949 UniProt P01040 P56567 RefSeq (mRNA) NM_005213 NM_029733.3 RefSeq (protein) NP_005204 NP_084009.1 Location (UCSC) Chr 3:
122.04 – 122.06 MbChr 16:
36.25 – 36.26 MbPubMed search [1] [2] Cystatin-A is a protein that in humans is encoded by the CSTA gene.[1][2]
The cystatin superfamily encompasses proteins that contain multiple cystatin-like sequences. Some of the members are active cysteine protease inhibitors, while others have lost or perhaps never acquired this inhibitory activity. There are three inhibitory families in the superfamily, including the type 1 cystatins (stefins), type 2 cystatins, and kininogens. This gene encodes a stefin that functions as a cysteine protease inhibitor, forming tight complexes with papain and the cathepsins B, H, and L. The protein is one of the precursor proteins of cornified cell envelope in keratinocytes and plays a role in epidermal development and maintenance. Stefins have been proposed as prognostic and diagnostic tools for cancer.[2]
Contents
Interactions
Cystatin A has been shown to interact with Cathepsin B[3][4] and CTSL1.[5][4]
References
- ^ Hsieh WT, Barrick JL, Berrettini WH, Chan MM, Fong D (Jun 1991). "A PstI DNA polymorphism in the human stefin A gene (STF 1)". Nucleic Acids Res 19 (7): 1722. doi:10.1093/nar/19.7.1722-a. PMC 333958. PMID 1674139. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=333958.
- ^ a b "Entrez Gene: CSTA cystatin A (stefin A)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1475.
- ^ Pavlova, Alona; Björk Ingemar (Sep. 2003). "Grafting of features of cystatins C or B into the N-terminal region or second binding loop of cystatin A (stefin A) substantially enhances inhibition of cysteine proteinases". Biochemistry (United States) 42 (38): 11326–33. doi:10.1021/bi030119v. ISSN 0006-2960. PMID 14503883.
- ^ a b Estrada, S; Nycander M, Hill N J, Craven C J, Waltho J P, Björk I (May. 1998). "The role of Gly-4 of human cystatin A (stefin A) in the binding of target proteinases. Characterization by kinetic and equilibrium methods of the interactions of cystatin A Gly-4 mutants with papain, cathepsin B, and cathepsin L". Biochemistry (UNITED STATES) 37 (20): 7551–60. doi:10.1021/bi980026r. ISSN 0006-2960. PMID 9585570.
- ^ Majerle, Andreja; Jerala Roman (Sep. 2003). "Protein inhibitors form complexes with procathepsin L and augment cleavage of the propeptide". Arch. Biochem. Biophys. (United States) 417 (1): 53–8. doi:10.1016/S0003-9861(03)00319-9. ISSN 0003-9861. PMID 12921779.
Further reading
- Järvinen M, Rinne A, Hopsu-Havu VK (1988). "Human cystatins in normal and diseased tissues--a review". Acta Histochem. 82 (1): 5–18. PMID 3122506.
- Brown WM, Dziegielewska KM (1997). "Friends and relations of the cystatin superfamily--new members and their evolution". Protein Sci. 6 (1): 5–12. doi:10.1002/pro.5560060102. PMC 2143511. PMID 9007972. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2143511.
- Kos J, Lah TT (1998). "Cysteine proteinases and their endogenous inhibitors: target proteins for prognosis, diagnosis and therapy in cancer (review)". Oncol. Rep. 5 (6): 1349–61. PMID 9769367.
- Rinne A, Järvinen M, Räsänen O (1979). "A protein reminiscent of the epidermal SH-protease inhibitor occurs in squamous epithelia of man and rat". Acta Histochem. 63 (2): 183–92. PMID 107702.
- Räsänen O, Järvinen M, Rinne A (1979). "Localization of the human SH-protease inhibitor in the epidermis. Immunofluorescent studies". Acta Histochem. 63 (2): 193–6. PMID 107703.
- Rasmussen HH, van Damme J, Puype M, et al. (1993). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes". Electrophoresis 13 (12): 960–9. doi:10.1002/elps.11501301199. PMID 1286667.
- Madsen P, Rasmussen HH, Leffers H, et al. (1991). "Molecular cloning, occurrence, and expression of a novel partially secreted protein "psoriasin" that is highly up-regulated in psoriatic skin". J. Invest. Dermatol. 97 (4): 701–12. doi:10.1111/1523-1747.ep12484041. PMID 1940442.
- Hsieh WT, Fong D, Sloane BF, et al. (1991). "Mapping of the gene for human cysteine proteinase inhibitor stefin A, STF1, to chromosome 3cen-q21". Genomics 9 (1): 207–9. doi:10.1016/0888-7543(91)90241-6. PMID 2004763.
- Rinne A, Järvinen M, Dorn A, et al. (1986). "[Low-molecular cysteine protease inhibitors in the human palatal tonsil]". Anatomischer Anzeiger 161 (3): 215–30. PMID 2424340.
- Kartasova T, Cornelissen BJ, Belt P, van de Putte P (1987). "Effects of UV, 4-NQO and TPA on gene expression in cultured human epidermal keratinocytes". Nucleic Acids Res. 15 (15): 5945–62. doi:10.1093/nar/15.15.5945. PMC 306060. PMID 2442723. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=306060.
- Takeda A, Kaji H, Nakaya K, et al. (1989). "Comparative studies on the primary structure of human cystatin as from epidermis, liver, spleen, and leukocytes". J. Biochem. 105 (6): 986–91. PMID 2768224.
- Strauss M, Stollwerk J, Lenarcic B, et al. (1989). "Chemical synthesis of a gene for human stefin A and its expression in E. coli". Biol. Chem. Hoppe-Seyler 369 (9): 1019–30. PMID 3067731.
- Davies ME, Barrett AJ (1984). "Immunolocalization of human cystatins in neutrophils and lymphocytes". Histochemistry 80 (4): 373–7. doi:10.1007/BF00495420. PMID 6429090.
- Machleidt W, Borchart U, Fritz H, et al. (1984). "Protein inhibitors of cysteine proteinases. II. Primary structure of stefin, a cytosolic protein inhibitor of cysteine proteinases from human polymorphonuclear granulocytes". Hoppe-Seyler's Z. Physiol. Chem. 364 (11): 1481–6. PMID 6689312.
- Söderström KO, Laato M, Wu P, et al. (1995). "Expression of acid cysteine proteinase inhibitor (ACPI) in the normal human prostate, benign prostatic hyperplasia and adenocarcinoma". Int. J. Cancer 62 (1): 1–4. doi:10.1002/ijc.2910620102. PMID 7541394.
- Tate S, Ushioda T, Utsunomiya-Tate N, et al. (1995). "Solution structure of a human cystatin A variant, cystatin A2-98 M65L, by NMR spectroscopy. A possible role of the interactions between the N- and C-termini to maintain the inhibitory active form of cystatin A". Biochemistry 34 (45): 14637–48. doi:10.1021/bi00045a004. PMID 7578072.
- Martin JR, Craven CJ, Jerala R, et al. (1995). "The three-dimensional solution structure of human stefin A". J. Mol. Biol. 246 (2): 331–43. doi:10.1006/jmbi.1994.0088. PMID 7869384.
- Steinert PM, Marekov LN (1997). "Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope". J. Biol. Chem. 272 (3): 2021–30. doi:10.1074/jbc.272.3.2021. PMID 8999895.
External Links
PDB gallery 1cyu: SOLUTION NMR STRUCTURE OF RECOMBINANT HUMAN CYSTATIN A UNDER THE CONDITION OF PH 3.8 AND 310K1cyv: SOLUTION NMR STRUCTURE OF RECOMBINANT HUMAN CYSTATIN A UNDER THE CONDITION OF PH 3.8 AND 310K1dvc: SOLUTION NMR STRUCTURE OF HUMAN STEFIN A AT PH 5.5 AND 308K, NMR, MINIMIZED AVERAGE STRUCTURE1dvd: SOLUTION NMR STRUCTURE OF HUMAN STEFIN A AT PH 5.5 AND 308K, NMR, 17 STRUCTURES1gd3: refined solution structure of human cystatin A1gd4: SOLUTION STRUCTURE OF P25S CYSTATIN A1n9j: Solution Structure of the 3D domain swapped dimer of Stefin A1nb3: Crystal structure of stefin A in complex with cathepsin H: N-terminal residues of inhibitors can adapt to the active sites of endo-and exopeptidases1nb5: Crystal structure of stefin A in complex with cathepsin HCategories:- Human proteins
- Chromosome 3 gene stubs
Wikimedia Foundation. 2010.