- Methyl jasmonate
-
Methyl jasmonate 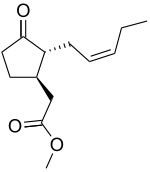 Methyl (1R,2R)-3-Oxo-2-(2Z)-2-
Methyl (1R,2R)-3-Oxo-2-(2Z)-2-
pentenyl-cyclopentaneacetateOther namesMethyl jasmonateIdentifiers CAS number 39924-52-2 
Jmol-3D images Image 1 - O=C1[C@H](C/C=C\CC) [C@@H](CC(OC)=O)CC1
Properties Molecular formula C13H20O3 Molar mass 224.3 g/mol Appearance Colorless liquid Melting point <25 °C
Boiling point 88-90 °C at 0.1 mmHg
 jasmonate (verify) (what is:
jasmonate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl jasmonate (MeJA) is a volatile organic compound used in plant defense and many diverse developmental pathways such as seed germination, root growth, flowering, fruit ripening, and senescense.[1] Methyl jasmonate is derived from jasmonic acid and the reaction is catalyzed by S-adenosyl-L-methionine:jasmonic acid carboxyl methyltransferase.[2] Plants produce jasmonic acid and methyl jasmonate in response to many biotic and abiotic stresses (in particular, herbivory and wounding), which build up in the damaged parts of the plant. The methyl jasmonate can be used to signal the original plant’s defense systems or it can be spread by physical contact or through the air to produce a defensive reaction in unharmed plants. The unharmed plants absorb the airborne MeJA through either the stomata or diffusion through the leaf cell cytoplasm. An herbivorous attack on a plant causes it to produce MeJA both for internal defense and for a signaling compound to other plants.[3]
MeJA can induce the plant to produce multiple different types of defense chemicals such as photoalexins (antimicrobial),[4] nicotine or proteinase inhibitors.[3] MeJA activates the proteinase inhibitor genes (a defensive reaction within plants) through a receptor-mediated signal transduction pathway.[5] The proteinase inhibitors interfere with the insect digestive process and discourage the insect from eating the plant again.[6]
MeJA has been used to stimulate traumatic resin duct production in lodgepole pine trees. This can be used as a defense against many insect attackers as a type of vaccine.
MeJA is also a plant hormone involved in tendril (root) coiling, flowering, seed and fruit maturation. An increase of the hormone affects flowering time, flower morphology and the number of open flowers.[7] MeJA induces ethylene-forming enzyme activity, which increases the amount of ethylene to the amount necessary for fruit maturation.[8]
Increased amounts of methyl jasmonate in plant roots have shown to inhibit their growth.[9] It is predicted that the higher amounts of MeJa activate previously unexpressed genes within the roots to cause the growth inhibition.[8]
Methyl jasmonate induces cytochrome c release in the mitochondria of cancer cells, leading to cell death, but does not harm normal cells. To be specific, it can cause cell death in chronic lymphocytic leukemia (CLL) cells taken from human patients with this disease and then treated in tissue culture with methyl jasmonate. Treatment of isolated normal human blood lymphocytes did not result in cell death [10]
See also
References
- ^ Cheong, Jong Joo and Yang Do Choi. [http://www.aloj.us.es/bioqplantas/tema9-11/biblio%20hormonas/Jasmonato.pdf, Trends in Genetics, 2003. Retrieved on 2010-10-27.
- ^ 7 April 2010. Retrieved on 2010-10-27.
- ^ a b Tao Xu et al. Chinese Science Bulletin, 2003. Retrieved on 2010-10-27.
- ^ [http://www.ars.usda.gov/is/ar/archive/feb98/fres0298.htm, United States Department of Agriculture, 1993. Retrieved on 2010-10-27.
- ^ Farmer, E. and C. Ryan Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves, 1990. Retrieved on 2010-10-27.
- ^ Tao Xu et al. Involvement of Jasmonate-signaling pathway in the herbivore-induced rice plant defense, 2003. Retrieved on 2010-10-27.
- ^ Radhika, V., J. Cost, W. Boland, and M. Heil. The role of jasmonates in floral nectar secretion. 2010. Retrieved on 2010-10-27.
- ^ a b Berger, S., E. Bell, and J. Mullet. Two Methyl Jasmonate-lnsensitive Mutants Show Altered Expression of AtVsp in Response to Methyl Jasmonate and Wounding, 1996. Retrieved on 2010-10-27.
- ^ Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development, 2007. Retrieved on 2010-10-27.
- ^ Rotem, R., A. Heyfets, O. Fingrut, D. Blickstein, M. Shaklai, and E. Flesher Jasmonates: novel anticancer agents acting directly and selectively on human cancer cell mitochondria. 2005. Retrieved on 2010-10-27.
External links
- General information about methyl jasmonate
- Jasmonates: novel anticancer agents acting directly and selectively on human cancer cell mitochondria.
- Jasmonate: pharmaceutical composition for treatment of cancer. US Patent Issued on October 22, 2002
- Plant stress hormones suppress the proliferation and induce apoptosis in human cancer cells, Leukemia, Nature, April 2002, Volume 16, Number 4, Pages 608-616
- Jasmonates induce nonapoptotic death in high-resistance mutant p53-expressing B-lymphoma cells, British Journal of Pharmacology (2005) 146, 800–808. doi:10.1038/sj.bjp.0706394; published online 19 September 2005
Plant hormones Brassinosteroids • Florigen • Jasmonates • Karrikins • Plant peptide hormones • Polyamine • Salicylic acid • StrigolactonesCategories:- Acetate esters
- Plant hormones
- Ketones
- Alkenes
Wikimedia Foundation. 2010.
