- Jasmonic acid
-
Jasmonic acid 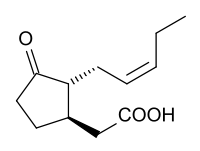 (1R,2R)-3-Oxo-2-(2Z)-2-pentenyl-cyclopentaneacetic acidOther namesJasmonic acid
(1R,2R)-3-Oxo-2-(2Z)-2-pentenyl-cyclopentaneacetic acidOther namesJasmonic acid
(-)-Jasmonic acid
JAIdentifiers CAS number 6894-38-8 
Jmol-3D images Image 1 - O=C1[C@H](C/C=C\CC)[C@@H](CC(O)=O)CC1
Properties Molecular formula C12H18O3 Molar mass 210.27 g/mol Density ? g/cm3 Boiling point 160 °C at 0.7 mmHg
 acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Jasmonic acid (JA) is derived from the fatty acid linolenic acid. It is a member of the jasmonate class of plant hormones. It is biosynthesized from linolenic acid by the octadecanoid pathway.
The major function of JA and its various metabolites is regulating plant responses to abiotic and biotic stresses as well as plant growth and development. [1] Regulated plant growth and development processes include growth inhibition, senescence, tendril coiling, flower development and leaf abscission. JA is also responsible for tuber formation in potatoes, yams, and onions. It has an important role in response to wounding of plants and systemic acquired resistance. When plants are attacked by insects, they respond by releasing JA, which activates the expression of protease inhibitors, among many other anti-herbivore defense compounds. These protease inhibitors prevent proteolytic activity of the insects' digestive proteases, thereby stopping them from acquiring the needed nitrogen in the protein for their own growth.[2]
Jasmonic acid is also converted to a variety of derivatives including esters such as methyl jasmonate; it may also be conjugated to amino acids.
This chemical may have a role in pest control, according to an October 2008 BBC News report.[3] In addition, researchers at the UK's Lancaster University have signed a licensing deal with an American company to market jasmonic acid as a spray to be applied to seeds prior to planting; such a spray has been found to stimulate the natural anti-pest defenses of the plants that germinate from the sprayed seeds, without harming plant growth as happens when the acid is sprayed on plants that have already started growing.[4]
References
- ^ Delker, C.; Stenzel, I.; Hause, B.; Miersch, O.; Feussner, I.; Wasternack, C. (2006). "Jasmonate Biosynthesis in Arabidopsis thaliana - Enzymes, Products, Regulation". Plant Biology 8 (3): 297–306. doi:10.1055/s-2006-923935. PMID 16807821.
- ^ Zavala, J. A.; Patankar, A. G.; Gase, K.; Hui, D.; Baldwin, I. T. (2004). "Manipulation of Endogenous Trypsin Proteinase Inhibitor Production in Nicotiana attenuata Demonstrates Their Function as Antiherbivore Defenses". Plant Physiology 134 (3): 1181–1190. doi:10.1104/pp.103.035634. PMC 389942. PMID 14976235. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=389942.
- ^ "Success for plants' pest control". BBC News. 2008-10-07. http://news.bbc.co.uk/1/hi/sci/tech/7656078.stm. Retrieved 2010-05-05.
- ^ "New UK tech protects crops without genetic modification". Cleantech.com. 2009-06-08. http://www.cleantech.com/news/4562/new-uk-technology-protects-crops-gm.
- Sankawa, Ushio; Barton, Derek H. R.; Nakanishi, Koji et al., eds (1999). Comprehensive Natural Products Chemistry : Polyketides and Other Secondary Metabolites Including Fatty Acids and Their Derivatives. Pergamon Press. ISBN 0-08-043153-4.
Plant hormones Brassinosteroids • Florigen • Jasmonates • Karrikins • Plant peptide hormones • Polyamine • Salicylic acid • StrigolactonesCategories:- Plant hormones
- Carboxylic acids
- Ketones
- Alkenes
Wikimedia Foundation. 2010.
