- Chalcone
-
- For the genus of grass skipper butterflies, see Chalcone (butterfly).
Chalcone[1] 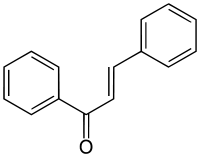 1,3-Diphenyl-2-propen-1-oneOther namesChalcone
1,3-Diphenyl-2-propen-1-oneOther namesChalcone
Chalkone
Benzylideneacetophenone
Phenyl styryl ketoneIdentifiers CAS number 94-41-7  ,
,
614-47-1 ((E)-Chalcone)PubChem 637760 ChemSpider 6921 
ChEBI CHEBI:27618 
Jmol-3D images Image 1 - O=C(C=Cc1ccccc1)c2ccccc2
Properties Molecular formula C15H12O Molar mass 208.26 g mol−1 Exact mass 208.088815 Density 1.071 g/cm3 Melting point 55–57 °C
Boiling point 345-348 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chalcone is an aromatic ketone and an enone that forms the central core for a variety of important biological compounds, which are known collectively as chalcones or chalconoids.
Contents
Chemical synthesis
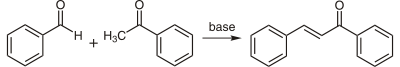
Chalcones can be prepared by an aldol condensation between a benzaldehyde and an acetophenone in the presence of sodium hydroxide as a catalyst.
This reaction has been found to work without any solvent at all - a solid-state reaction.[2] The reaction between substituted benzaldehydes and acetophenones has been used to demonstrate green chemistry in undergraduate chemistry education.[3] In a study investigating green chemistry synthesis, chalcones were also synthesized from the same starting materials in high temperature water (200 to 350 °C).[4]
Chemical reactions
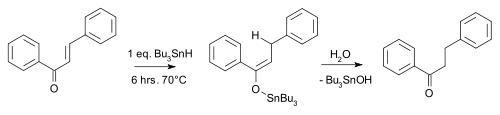
An example is the carbonyl reduction of chalcone by tributyltin hydride [5]:
Conjugate reduction chalcone An asymmetric version of this reaction has also been developed [6].
See also
- Juliá-Colonna epoxidation
References
- ^ Merck Index, 11th Edition, 2028.
- ^ Toda, F., et al., J. Chem. Soc. Perkin Trans. I, 1990, 3207.
- ^ Palleros, D. R., J. Chem. Educ., 81, 1345 (2004).
- ^ Comisar, C. M. and Savage, P. E. Green Chem., 6 (2004), 227 - 231. doi:10.1039/b314622g
- ^ Leusink, A.J.; Noltes, J.G. (1966). "Reaction of organotin hydrides with α,β-unsaturated ketones". Tetrahedron Letters 7 (20): 2221. doi:10.1016/S0040-4039(00)72405-1.
- ^ Moritani, Yasunori; Appella, Daniel H.; Jurkauskas, Valdas; Buchwald, Stephen L. (2000). "Synthesis of β-Alkyl Cyclopentanones in High Enantiomeric Excess via Copper-Catalyzed Asymmetric Conjugate Reduction". Journal of the American Chemical Society 122 (28): 6797. doi:10.1021/ja0009525.
External links
Categories:- Chalconoids
Wikimedia Foundation. 2010.