- Ethanol purification
-
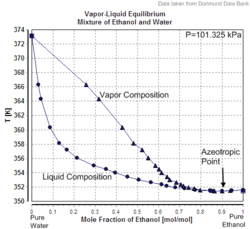 Vapor-liquid equilibrium of the mixture of Ethanol and Water (incl. azeotrope), composition given in molefractions
Vapor-liquid equilibrium of the mixture of Ethanol and Water (incl. azeotrope), composition given in molefractions
The product of either ethylene hydration or brewing is an ethanol-water mixture. For most industrial and fuel uses, the ethanol must be purified. Fractional distillation can concentrate ethanol to 95.6% by weight (89.5 mole%). The mixture of 95.6% ethanol and 4.4% water (percentage by weight) is an azeotrope with a boiling point of 78.2 °C, and cannot be further purified by distillation. Because of the difficulty of further purification, 95% ethanol/5% water is a fairly common solvent. There are several methods used to further purify ethanol beyond 95.6%:
Contents
Drying using lime or a salt
After distillation ethanol can be further purified by "drying" it using lime (calcium oxide) or using a hygroscopic material such as rocksalt. When lime is mixed with the water in ethanol, calcium hydroxide forms. The calcium hydroxide can then be separated from the ethanol. Similarly, a hygroscopic material will dissolve some of the water content of the ethanol as it passes through, leaving a purer alcohol.[1]
Addition of an entrainer
The ethanol-water azeotrope can be broken by the addition of a small quantity of benzene or cyclohexane. Benzene, ethanol, and water form a ternary azeotrope with a boiling point of 64.9 °C. Since this azeotrope is more volatile than the ethanol-water azeotrope, it can be fractionally distilled out of the ethanol-water mixture, extracting essentially all of the water in the process. The bottoms from such a distillation is anhydrous ethanol, with several parts per million residual benzene. Benzene is toxic to humans, and cyclohexane has largely supplanted benzene in its role as the entrainer in this process. However, this purification method leaves chemical residues which render the alcohol unfit for human consumption.
Molecular sieves
Alternatively, a molecular sieve can be used to selectively absorb the water from the 95.6% ethanol solution. Synthetic zeolite in pellet form can be used, as well as a variety of plant-derived absorbents, including cornmeal, straw, and sawdust. The zeolite bed can be regenerated essentially an unlimited number of times by drying it with a blast of hot carbon dioxide. Cornmeal and other plant-derived absorbents cannot readily be regenerated, but where ethanol is made from grain, they are often available at low cost. Absolute ethanol produced this way has no residual benzene, and can be used to fortify port and sherry in traditional winery operations.
Membranes
Membranes can also be used to separate ethanol and water. The membrane can break the water-ethanol azeotrope because separation is not based on vapor-liquid equilibria. Membranes are often used in the so-called hybrid membrane distillation process. This process uses a pre-concentration distillation column as first separating step. The further separation is then accomplished with a membrane operated either in vapor permeation or pervaporation mode. Vapor permeation uses a vapor membrane feed and pervaporation uses a liquid membrane feed.
Pressure Reduction
At pressures less than atmospheric pressure, the composition of the ethanol-water azeotrope shifts to more ethanol-rich mixtures, and at pressures less than 70 torr (9.333 kPa) , there is no azeotrope, and it is possible to distill absolute ethanol from an ethanol-water mixture. While vacuum distillation of ethanol is not presently economical, pressure-swing distillation is a topic of current research. In this technique, a reduced-pressure distillation first yields an ethanol-water mixture of more than 95.6% ethanol. Then, fractional distillation of this mixture at atmospheric pressure distills off the 95.6% azeotrope, leaving anhydrous ethanol at the bottoms.
References
- ^ Mathewson, S.W. (1980). "Drying the Alcohol". The Manual for the Home and Farm Production of Alcohol Fuel. Ten Speed Press. http://www.journeytoforever.org/biofuel_library/ethanol_manual/manual12.html. Retrieved 2006-07-01.
Categories:- Alcohols
- Ethanol fuel
Wikimedia Foundation. 2010.
