- Molisch's test
-
Molisch's Test (named after Austrian botanist Hans Molisch) is a sensitive chemical test for the presence of carbohydrates, based on the dehydration of the carbohydrate by sulfuric acid to produce an aldehyde, which condenses with two molecules of phenol (usually α-naphthol, though other phenols (e.g. resorcinol, thymol) also give colored products) resulting in a red- or purple-colored compound.
Procedure
The test solution is combined with a small amount of Molisch's reagent (α-naphthol dissolved in ethanol) in a test tube. After mixing, a small amount of concentrated sulfuric acid is slowly added down the sides of the sloping test-tube, without mixing, to form a bottom layer. A positive reaction is indicated by appearance of a purple ring at the interface between the acid and test layers.
Reaction
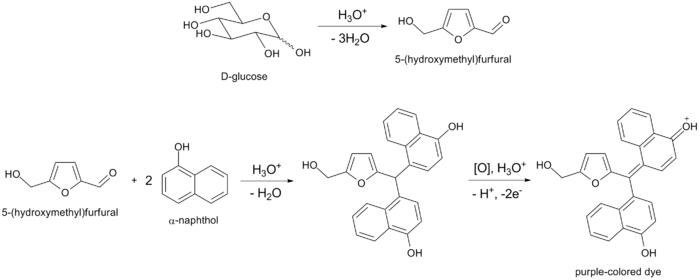
All carbohydrates -- monosaccharides, disaccharides, and polysaccharides -- should give a positive reaction, and nucleic acids and glycoproteins also give a positive reaction, as all these compounds are eventually hydrolyzed to monosacharides by strong mineral acids. Pentoses are then dehydrated to furfural, while hexoses are dehydrated to 5-hydroxymethylfurfural. Either of these aldehydes, if present, will condense with two molecules of naphthol to form a purple-colored product, as illustrated below by the example of glucose:
References
- Nachweisreaktion von Zuckern nach Molisch (Uni Regensburg) (in German, including a photo of the reaction tube, English version also available)
Categories:- Chemical tests
Wikimedia Foundation. 2010.