- 17-Hydroxyprogesterone caproate
-
17-Hydroxyprogesterone caproate 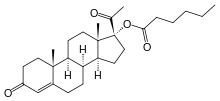
Systematic (IUPAC) name [(8R,9S,10R,13S,14S,17R)-17-acetyl-10,13-dimethyl-
3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-
1H-cyclopenta[a]phenanthren-17-yl] hexanoateClinical data Pregnancy cat. ? Legal status ? Identifiers CAS number 630-56-8 
ATC code ? PubChem CID 169870 ChemSpider 148552 
Chemical data Formula C27H40O4 Mol. mass 428.6041 g/mol SMILES eMolecules & PubChem  (what is this?) caproate (verify)
(what is this?) caproate (verify)17α-Hydroxyprogesterone caproate is a synthetic steroid hormone that is similar to medroxyprogesterone acetate and megestrol acetate. It is an ester derivative of 17α-hydroxyprogesterone formed from caproic acid (hexanoic acid).
17α-Hydroxyprogesterone caproate was previously marketed under the trade name Delalutin by Squibb, which was approved by the U.S. Food and Drug Administration (FDA) in 1956 and withdrawn from marketing in 1999.
The US FDA approved Makena from KV Pharmaceutical (previously named as Gestiva) on February 4, 2011 for prevention of preterm delivery in women with a history of preterm delivery, sparking a pricing controversy.
Contents
Safety
The use of 17-hydroxyprogesterone caproate in pregnancy to prevent preterm birth is not recommended without further study according to two authorities. A 2006 Cochrane Review concluded "...important maternal and infant outcomes have been poorly reported to date... information regarding the potential harms of progesterone therapy to prevent preterm birth is limited".[1] There was a similar conclusion from a review by Marc Keirse of Flinders University.[2] Three clinical studies in singleton pregnancies of 250 mg/week of intramuscular 17-hydroxyprogesterone caproate have all shown a trend for an increase in pregnancy loss due to miscarriage compared to placebo.[3][4][5][6] The FDA expressed concern about miscarriage at the 2006 advisory committee meeting; the committee voted unanimously that further study was needed to evaluate the potential association of 17OHP-C with increased risk of second trimester miscarriage and stillbirth.[7] A toxicology study in rhesus monkeys resulted in the death of all rhesus fetuses exposed to 1 and 10 times the human dose equivalent of 17-hydroxyprogesterone caproate.[8] as of 2008[update], 17-hydroxyprogesterone caproate is a category D progestin according to the FDA (that is, there is evidence of fetal harm). There is speculation that the castor oil in the 17-hydroxyprogesterone caproate formulation may not be beneficial for pregnancy.[9][10]
Synthesis
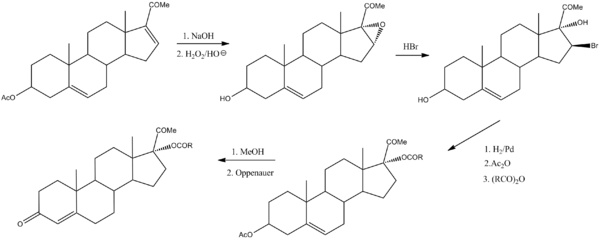
Hydroxyprogesterone caproate can be prepared by the following sequence:[11]
Makena
A 2011 decision by the USFDA was going to result in driving "up the [US] cost of an available medication from about $300 to $30,000 — about a 100-fold increase — with minimal added clinical benefit".[12] However, the USFDA said it would not go after compounding pharmacies that filled prescriptions, and KV Pharmaceutical announced a lower price.[12]
Notes
- ^ Dodd JM, Flenady V, Cincotta R, Crowther CA; The Cochrane Database of Systematic Reviews 2006 Issue 1
- ^ Keirse MJNC. Progesterone and preterm: seventy years of "déjà vu" or "still to be seen"?. Birth, 2004 September; 31:3.
- ^ Johnson JWC, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of premature labor. NEJM 1975 October. 293(14):675.
- ^ Yemini M, Borenstein R, Dreazen, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151(5):574-7.
- ^ Meis PJ et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-hydroxyprogesterone Caproate. NEJM, 2003: vol 348, no 24, pg 2379-2385.
- ^ Keirse MJNC, Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynecol 1990 February; 97:149.
- ^ Advisory Committees: CDER 2006 Meeting Documents
- ^ Hendrix AG, et al. Embriotoxicity of sex steroidal hormones in nonhuman primates: II. Hydroxyprogesterone caproate, estradiol valerate. Teratology 1987 February. 35 (1): 129.
- ^ Duke University Medical Center, New England Journal of Medicine, correspondence, vol 349.
- ^ Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983 May; 146(2): 187.
- ^ Ringold, H. J.; Loken, B.; Rosenkraz, G.; Sondheimer, F. (1956). J. Amer. Chem. Soc. 78 (4): 816. doi:10.1021/ja01585a030.
- ^ a b Armstrong J (May 2011). "Unintended consequences — the cost of preventing preterm births after FDA approval of a branded version of 17OHP". N. Engl. J. Med. 364 (18): 1689–91. doi:10.1056/NEJMp1102796. PMID 21410391. http://www.nejm.org/doi/full/10.1056/NEJMp1102796.
Sources
- FDA Reproductive Health Drugs Advisory Committee. August 29, 2006 Meeting to discuss NDA 21-945 Gestiva (Adeza Biomedical)
17α-hydroxyprogesterone caproate injection, 250 mg/mL, for the proposed indication: prevention of preterm delivery in women with a history of a prior preterm delivery. - Adeza Biomedical (October 23, 2006) Receives FDA Approvable Letter For Gestiva
- Adeza gets orphan drug designation for Gestiva (January 31, 2007)
- Cytyc Acquires Adeza Biomedical Corporation (April 3, 2007)
Adeza's name changed to Cytyc Prenatal Products Corp.
Categories:- Steroid hormones
Wikimedia Foundation. 2010.
