- Mosher's acid
-
Mosher's acid 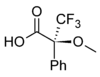

 (R/S)-3,3,3-trifluoro-2-
(R/S)-3,3,3-trifluoro-2-
methoxy-2-phenylpropanoic acidOther namesMethoxy(trifluoromethyl)phenylacetic acid, MTPAIdentifiers CAS number 81655-41-6, (racemic)
[20445-31-2] (R)
[17257-71-5] (S)Jmol-3D images Image 1 - COC(C(O)=O)(c1ccccc1)C(F)(F)F
Properties Molecular formula C10H9F3O3 Molar mass 234.17 Appearance solid Melting point 46-49°C (319-322 K)
Boiling point 105 - 107 °C at 1 torr
Hazards R-phrases R36/37/38 S-phrases Template:S26-36 Flash point 110°C Related compounds Related ? Mosher's acid chloride  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mosher's acid, or α-methoxy-α-trifluoromethylphenylacetic acid (MTPA) is a carboxylic acid which was first used by Harry Stone Mosher as a chiral derivatizing agent.[1][2][3][4] It is a chiral molecule, consisting of R and S enantiomers.
Applications
As a chiral derivatizing agent, it reacts with an alcohol or amine[5] of unknown stereochemistry to form an ester or amide. The absolute configuration of the ester or amide is then determined by proton and/or 19F NMR spectroscopy.
Mosher's acid chloride, the acid chloride form, is sometimes used because it has better reactivity.[6]
References
- ^ J. A. Dale, D. L. Dull, H. S. Mosher (1969). "α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines". Journal of Organic Chemistry 34 (9): 2543–2549. doi:10.1021/jo01261a013.
- ^ J. A. Dale, H. S. Mosher (1973). "Nuclear magnetic resonance enantiomer regents. Configurational correlations via nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, and α-methoxy-α-trifluoromethylphenylacetate (MTPA) esters". Journal of the American Chemical Society 95 (2): 512–519. doi:10.1021/ja00783a034.
- ^ Y. Goldberg, H. Alper (1992). "A new and simple synthesis of Mosher's acid". Journal of Organic Chemistry 57 (13): 3731–3732. doi:10.1021/jo00039a043.
- ^ D. L. Dull, H. S. Mosher (1967). "Aberrant rotatory dispersion curves of α-hydroxy- and α-methoxy-α-trifluoromethylphenylacetic acids". Journal of the American Chemical Society 89 (16): 4230–4230. doi:10.1021/ja00992a053.
- ^ See for example: Mosher Amides: Determining the Absolute Stereochemistry of Optically-Active Amines Allen, Damian A.; Tomaso, Anthony E., Jr.; Priest, Owen P.; Hindson, David F.; Hurlburt, Jamie L. J. Chem. Educ. 2008, 85, 698. Abstract
- ^ D. E. Ward, C. K. Rhee (1991). "A simple method for the microscale preparation of Mosher's acid chloride". Tetrahedron Letters 32 (49): 7165–7166. doi:10.1016/0040-4039(91)80466-J.
Categories:- Stereochemistry
- Carboxylic acids
- Organofluorides
Wikimedia Foundation. 2010.
