- Cubical atom
-
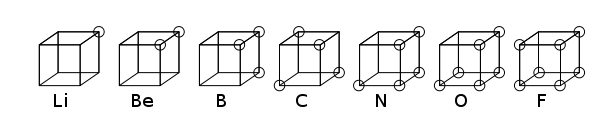
The cubical atom was an early atomic model in which electrons were positioned at the eight corners of a cube in a non-polar atom or molecule. This theory was developed in 1902 by Gilbert N. Lewis and published in 1916 in the famous article "The Atom and the Molecule" and used to account for the phenomenon of valency.[1] Lewis's theory was based on Abegg's rule. It was further developed in 1919 by Irving Langmuir as the cubical octet atom.[2] The figure below shows structural representations for elements of the second row of the periodic table.
Although the cubical model of the atom was soon abandoned in favor of the quantum mechanical model based on the Schrödinger equation, and is therefore now principally of historical interest, it represented an important step towards the understanding of the chemical bond. The 1916 article by Lewis also introduced the concept of the electron pair in the covalent bond, the octet rule, and the now-called Lewis structure.
Bonding in the cubical atom model
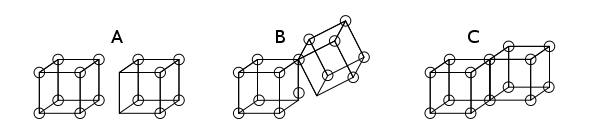
Single covalent bonds are formed when two atoms share an edge, as in structure C below. This results in the sharing of two electrons. Ionic bonds are formed by the transfer of an electron from one cube to another, without sharing an edge (A). An intermediate state B where only one corner is shared was also postulated by Lewis.
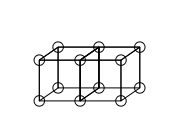
Double bonds are formed by sharing a face between two cubic atoms. This results in sharing four electrons:
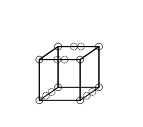
Triple bonds could not be accounted for by the cubical atom model, because there is no way of having two cubes share three parallel edges. Lewis suggested that the electron pairs in atomic bonds have a special attraction, which result in a tetrahedral structure, as in the figure below (the new location of the electrons is represented by the dotted circles in the middle of the thick edges). This allows the formation of a single bond by sharing a corner, a double bond by sharing an edge, and a triple bond by sharing a face. It also accounts for the free rotation around single bonds and for the tetrahedral geometry of methane. Remarkably, it could be said that there was a grain of truth in this idea, because it was later shown that the Pauli exclusion principle results in a "Fermi hole" of decreased repulsion between a pair of electrons with opposite spins in the same orbital.
See also
References
- ^ Lewis, Gilbert N. (1916-04-01). "The Atom and the Molecule.". Journal of the American Chemical Society 38 (4): 762–785. doi:10.1021/ja02261a002. http://osulibrary.oregonstate.edu/specialcollections/coll/pauling/bond/papers/corr216.3-lewispub-19160400.html. See images of original article http://chimie.scola.ac-paris.fr/sitedechimie/hist_chi/text_origin/lewis/Lewis-1916.htm
- ^ Langmuir, Irving (1919-06-01). "The Arrangement of Electrons in Atoms and Molecules.". Journal of the American Chemical Society 41 (6): 868–934. doi:10.1021/ja02227a002.
Atomic Models Single atoms Dalton model (Billiard Ball Model) · Thomson model (Plum Pudding Model) · Lewis model (Cubical Atom Model) · Nagaoka model (Saturnian Model) · Rutherford model (Planetary Model) · Bohr model (Rutherford–Bohr Model) · Bohr–Sommerfeld model (Refined Bohr Model) · Gryziński model (Free-fall Model) · Schrodinger model (Electron Cloud Model)Atoms in solids Atoms in liquids Atoms in gases Scientists  Book:Atomic models ·
Book:Atomic models ·  Category:Atoms ·
Category:Atoms ·  Portal:Physics / ChemistryCategories:
Portal:Physics / ChemistryCategories:- Obsolete scientific theories
- Atoms
- Chemical bonding
Wikimedia Foundation. 2010.