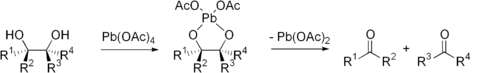- Glycol cleavage
-
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a (vicinal diol (glycol) is cleaved and replaced with two carbon–oxygen double bonds. Depending on the substitution pattern in the diol, either ketones or aldehydes may be formed.
Glycol cleavage is an important reaction in the laboratory because it is useful for determining the structures of sugars. After cleavage takes place the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained.[1]
Reagents
Periodic acid (HIO4) and lead tetraacetate (Pb(OAc)4) are the most common reagents used for glycol cleavage. These reactions involve cyclic intermediates which then decompose to form ketones (if the R groups are not H) or aldehydes (if one of the R groups is H).
Warm concentrated potassium permanganate (KMnO4) will react with an alkene to form a glycol and will then immediately cleave the glycol to give stable ketones or oxidizable aldehydes. The aldehydes will react further to become carboxylic acids. Controlling the temperature and concentration of the reagent can keep the reaction from continuing past the formation of the glycol.
Glycol cleavage by periodic acid is called Malaprade periodic acid oxidation first reported by M. Malaprade in 1934 [2] and also works with alpha aminoalcohols [3]References
- ^ Wade, L. G. Organic Chemistry, 6th ed.., Prentice Hall, Upper Saddle River, New Jersey, 2005; pp 358–361, pp 489–490. ISBN 0131478826
- ^ L. Malaprade, Bull. Soc. Chim. Fr. 3, 1, 833 1934;
- ^ THE ACTION OF PERIODIC ACID ON α-AMINO ALCOHOLS Ben H. Nicolet, Leo A. Shinn J. Am. Chem. Soc., 1939, 61 (6), p 1615 doi:10.1021/ja01875a521
External links
Categories:- Organic redox reactions
-
Wikimedia Foundation. 2010.