N,N'-Di-2-butyl-1,4-phenylenediamine
- N,N'-Di-2-butyl-1,4-phenylenediamine
-
| N,N'-Di-2-butyl-1,4-phenylenediamine |
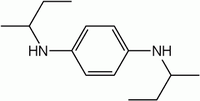 |
N,N'-Di-sec-butyl-benzene-1,4-diamine
|
Other names
N,N′-di-sec-butyl-1,4-phenylenediamine, N,N'-bis(1-methylpropyl)-1,4-benzenediamine
|
| Identifiers |
| CAS number |
101-96-2 |
| Jmol-3D images |
Image 1 |
|
|
| Properties |
| Molecular formula |
C14H24N2 |
| Molar mass |
220.354 g/mol |
 Y (verify) (what is: Y (verify) (what is:  Y/ Y/ N?) N?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
| Infobox references |
N,N'-Di-2-butyl-1,4-phenylenediamine is an aromatic amine used industrially as an antioxidant to prevent degradation of turbine oils, transformer oils, hydraulic fluids, lubricants, waxes, and greases. It is particularly effective for hydrocarbon products produced by cracking or pyrolysis, which are characterized by high olefin content. It is also used as an inhibitor in production of various vinyl monomers.
It has the appearance of a corrosive red liquid. It is a skin sensitizer and can be absorbed through skin. It is toxic.
It is the active component of eg. AO-22, AO-24, AO-29, and VANLUBE antioxidant mixtures, and Santoflex 44PD inhibitor.
Wikimedia Foundation.
2010.
Look at other dictionaries:
Antioxidant — Model of the antioxidant metabolite glutathione. The yellow sphere is the redox active sulfur atom that provides antioxidant activity, while the red, blue, white, and dark grey spheres represent oxygen, nitrogen, hydrogen, and carbon atoms,… … Wikipedia
Diamine — Une diamine est un type de polyamine contenant exactement deux groupes amine. Les diamines sont principalement utilisées comme monomères pour synthétiser des polyamides, des polyimides et des polyurées. La principale diamine produite est le 1,6… … Wikipédia en Français
Diamine — A diamine is a type of polyamine with exactly two amino groups. Diamines are mainly used as monomers to prepare polyamides, polyimides and polyureas. In terms of quantities produced, 1,6 diaminohexane, a precursor to Nylon 6 6, is most important … Wikipedia
List of IARC Group 3 carcinogens — Substances, mixtures and exposure circumstances in this list have been classified by the IARC as Group 3: The agent (mixture or exposure circumstance) is not classifiable as to its carcinogenicity to humans. This category is used most commonly… … Wikipedia
Liste des cancérogènes du groupe 3 du CIRC — Cette liste énumère toutes les substances, mélanges et circonstances d’exposition évaluées à ce jour et classées dans le Groupe 3 (inclassables quant à leur cancérogénicité pour l Homme) du CIRC. Sommaire 1 Agents et groupes d agents 2 Mélanges 3 … Wikipédia en Français
ANTIOXYGÈNES — Le terme «antioxygène» désigne des substances qui, ajoutées à faible dose à des matières spontanément oxydables à l’air, sont capables d’empêcher l’action de l’oxygène libre, communément appelée autoxydation. Du point de vue de la terminologie,… … Encyclopédie Universelle
List of organic compounds — This page aims to list well known organic compounds, including organometallic compounds, to stimulate the creation of Wikipedia articles. Note that purely inorganic compounds, minerals, and chemical elements are not included on this list. There… … Wikipedia
carboxylic acid — Chem. any organic acid containing one or more carboxyl groups. [1900 05; CARBOXYL + IC] * * * Any organic compound with the general chemical formula ―COOH in which a carbon (C) atom is bonded to an oxygen (O) atom by a double bond to make a… … Universalium
National Emissions Standards for Hazardous Air Pollutants — The National Emissions Standards for Hazardous Air Pollutants (NESHAPs) are emissions standards set by the United States EPA for an air pollutant not covered by NAAQS that may cause an increase in fatalities or in serious, irreversible, or… … Wikipedia
Liste des cancérogènes du groupe 2B du CIRC — La liste des cancérogènes du groupe 2B du CIRC répertorie toutes les substances, mélanges et circonstances d’exposition déjà évaluées et classées dans le Groupe 2B, celui des cancérogènes possibles pour l homme, par le Centre international de… … Wikipédia en Français
 N,N'-Di-sec-butyl-benzene-1,4-diamineOther namesN,N′-di-sec-butyl-1,4-phenylenediamine, N,N'-bis(1-methylpropyl)-1,4-benzenediamine
N,N'-Di-sec-butyl-benzene-1,4-diamineOther namesN,N′-di-sec-butyl-1,4-phenylenediamine, N,N'-bis(1-methylpropyl)-1,4-benzenediamine (verify) (what is:
(verify) (what is:  /
/ ?)
?)