- Deactivating groups
-
In organic chemistry, a deactivating group (or electron withdrawing group) is a functional group attached to a benzene molecule that removes electron density from the benzene ring, making electrophilic aromatic substitution reactions slower and more complex relative to benzene. Depending on their relative strengths, deactivating groups also determine the positions (relative to themselves) on the benzene ring where substitutions must take place; this property is therefore important in processes of organic synthesis.Contents
Categories
Deactivating groups are generally sorted into three categories. Weakly deactivating groups direct electrophiles to attack the benzene molecule at the ortho- and para- positions, while strongly and moderately deactivating groups direct attacks to the meta- position. This is not a case of favouring the meta- position like para- and ortho- directing functional groups, but rather disfavouring the para- and ortho- positions more than they disfavour the meta- position.
Strongly Deactivating Groups
-NO2, nitro groups
-NR3+ Quaternary amine /Quaternary ammonium base
-CF3, CCl3 trihalides
Moderately Deactivating Groups
-CN Cyano Groups
-SO3H Sulfonates
-COOH Carboxylic acid
-COOR Esters
-CHO, -COR Aldehydes and Ketones
Halides are ortho- para- directing groups due to the lone pair activating the benzene ring. F directs strongly to the para position (86%) while I directs to ortho and para (45% and 54% respectively).
Mechanism
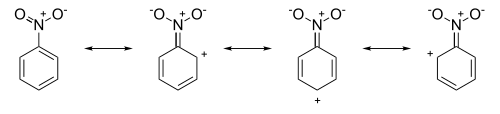
While steric effects are a consideration, the major contribution of deactivating groups is achieved by utilizing the nature of conjugated systems (specifically the ease through which mesomeric effects travel through such systems) to create regions of positive charge within the resonance contributors. For example, in nitrobenzene the resonance structures have positive charges around the ring system:
The resulting resonance hybrid, now possessing δ+ charges in the ortho- and para- positions repels approaching electrophiles increasing the relative success of attack in the meta position.
The selectivities observed with activating groups and deactivating groups were first described in 1892 and have been known as the Crum Brown-Gibson Rule.[1][2]
See also
References
Categories:- Functional groups
Wikimedia Foundation. 2010.