- Methylene
-
Methylene 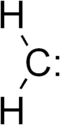 Methylene[citation needed]Systematic nameOther names
Methylene[citation needed]Systematic nameOther namesIdentifiers CAS number 2465-56-7 PubChem 123164 ChemSpider 109779 
MeSH carbene ChEBI CHEBI:29357 
Beilstein Reference 1696832 Gmelin Reference 56 Jmol-3D images Image 1 - [CH2]
Properties Molecular formula CH22• Molar mass 14.0266 g mol-1 Exact mass 14.015650064 g mol-1 Appearance Colourless gas Solubility in water Reacts Thermochemistry Std enthalpy of
formation ΔfHo298386.39 kJ mol-1 Standard molar
entropy So298193.93 J K-1 mol-1 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Methylene is a carbene encountered in organic chemistry.[2][3] Methylene has a non-linear triplet ground state and is thus paramagnetic. It is stable in the gaseous state. Methylene can be considered to be a diradical; addition reactions are very fast and exothermic. Methylene tends to dimerize at high concentrations into ethene.[4]
Trivia
Carl Barks was the first to make a reference to methylene in his comic Donald Duck, almost 20 years before science could prove its existence.[5]
References
- ^ a b "methanediyl (CHEBI:29357)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. IUPAC Name. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI%3A29357.
- ^ Hoffmann, Roald (2005). Molecular Orbitals of Transition Metal Complexes. Oxford. p. 7. ISBN 0198530935
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carbenes".
- ^ Lazár, Milan; Lazr̀, Milan (1989). Free radicals in chemistry and biology. Boca Raton: CRC Press. ISBN 0-8493-5387-4.
- ^ http://www.cracked.com/article_19021_5-amazing-things-invented-by-donald-duck-seriously_p2.html
Further reading
- Shavitt, I (1985). "Geometry and singlet-triplet energy gap in methylene: A critical review of experimental and theoretical determinations". Tetrahedron 41 (8): 1531. doi:10.1016/S0040-4020(01)96393-8.

This article about a hydrocarbon is a stub. You can help Wikipedia by expanding it.
