- Pulegone
-
Pulegone[1] 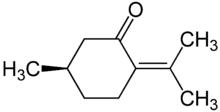 (R)-5-Methyl-2-(1-methylethylidine)cyclohexanoneOther namesp-Menth-4(8)-en-3-one;
(R)-5-Methyl-2-(1-methylethylidine)cyclohexanoneOther namesp-Menth-4(8)-en-3-one;
δ-4(8)-p-menthen-3-one;
(R)-2-Isopropylidene-5-methylcyclohexanone;
(R)-p-Menth-4(8)-en-3-one;
(R)-(+)-PulegoneIdentifiers CAS number 89-82-7 
PubChem 442495 ChemSpider 390923 
UNII 4LF2673R3G 
ChEBI CHEBI:35596 
Jmol-3D images Image 1 - O=C1/C(=C(/C)C)CC[C@@H](C)C1
Properties Molecular formula C10H16O Molar mass 152.23 g/mol Appearance Colorless oil Density 0.9346 g/cm3 Boiling point 224 °C, 497 K, 435 °F
Solubility in water Insoluble Solubility in Ethanol
Ether
ChloroformMiscible Hazards MSDS MSDS[2]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal.[3][4] It is classified as a monoterpene.
Pulegone is a clear colorless oily liquid and has a pleasant odor similar to pennyroyal, peppermint and camphor. It is used in flavoring agents, in perfumery, and in aromatherapy.
Contents
Toxicology
It was reported that the chemical is toxic to rats if a large quantity is consumed.[5] Asekun et al. found that the chemical content of Mentha longifolia L was decreased by the treatments at high temperatures, suggesting that the herb should be oven dried or thoroughly cooked before consumption.[6]
Plants that contain the chemical
Notes and references
- ^ Merck Index, 11th Edition, 7955.
- ^ Universiti Malaysia Pahang. "Safety data sheet". http://notes.ump.edu.my/fkksa/FKKSA/Archive/Technical%20Unit/Warehouse%20Unit/Chemical/MSDS/MERCK_EN/8186/818665.pdf. Retrieved 8 June 2009.
- ^ Grundschober, F. (1979) Literature review of pulegone. Perfum. Flavorist, 4, 15–17.
- ^ Sullivan, J.B., Rumack, B.H., Thomas, H., Peterson, R.G. & Brysch, P. (1979) Pennyroyal oil poisoning and hepatotoxicity. J. Am. Med. Assoc., 242, 2873–2874.
- ^ Thorup, I. et al.; Würtzen, G; Carstensen, J; Olsen, P (1983). "Short term toxicity study in rats dosed with pulegone and menthol". Toxicology Letters 19 (3): 207–210. doi:10.1016/0378-4274(83)90120-0. PMID 6658833.
- ^ a b Asekun, O.T. et al.; Grierson, D; Afolayan, A (2006). "Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. Capensis". Food Chemistry 101 (3): 995–998. doi:10.1016/j.foodchem.2006.02.052.
- ^ Gordon, W. Perry et al.; Valerie Howland (1982). "Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse". Toxicology and Applied Pharmacology 65 (3): 413–424. doi:10.1016/0041-008X(82)90387-8. PMID 7157374.
- ^ Farley, Derek R.; Valerie Howland (2006). "The natural variation of the pulegone content in various oils of peppermint". Journal of the Science of Food and Agriculture 31 (11): 1143–1151. doi:10.1002/jsfa.2740311104.
See also
- Cerebellum
- Creatinine
- Doebner-Miller reaction
- Isopulegone
- Menthofuran
- (+)-Menthofuran
- Menthol
- Pennyroyal
- (−)-Pulegone
Categories:- Ketones
- Flavors
- Perfume ingredients
- Monoterpenes
Wikimedia Foundation. 2010.
