- Potassium hydrogenoxalate
-
Potassium hydrogenoxalate 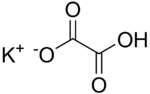 Potassium 2-hydroxy-2-oxoacetateOther namesPotassium bioxalate
Potassium 2-hydroxy-2-oxoacetateOther namesPotassium bioxalateIdentifiers CAS number 127-95-7 PubChem 23662386 Jmol-3D images Image 1 - C(=O)(C(=O)[O-])O.[K+]
Properties Molecular formula C2HKO4 Molar mass 128.13 g mol−1 Appearance White crystalline solid Solubility in water 2.5 g/100 g  hydrogenoxalate (verify) (what is:
hydrogenoxalate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Potassium hydrogenoxalate, also known as potassium bioxalate, is a salt with formula KHC2O4 or K+·HO2C-CO2−. It is one of the most common salts of the hydrogenoxalate anion, and can be obtained by reacting potassium hydroxide with oxalic acid in 1:1 mole ratio.
The salt is also known as potassium hydrogen oxalate, potassium binoxalate, acid potassium oxalate, or monobasic potassium oxalate. In older literature, it was also called sorrel salt, sal acetosella, or (rather improperly) salt of lemon
Potassium hydrogenoxalate occurs in some plants, notably sorrel. It is a commercial product, used in photography, marble grinding, and to remove ink stains.
Contents
Properties
The anhydrous product is a white, odorless, crystalline solid, hygroscopic and soluble in water (2.5 g/100 g at room temperature). The solutions are basic. Below 50 °C the much less soluble potassium tetraoxalate forms and precipitates out of solution.[1]
The monohydrate KHC2O4·H2O starts losing the water at 100 °C.[2]
The anhydrous salt was found to have remarkable elastic anisotropy, due to its crystal structure that consists of relatively rigid columns of hydrogen-bonded hydrogenoxalate anions, joined into sheets by ionic K–O bonds.[3]
Toxicity
Potassium hydrogenoxalate is strongly irritating to eyes, mucoses and gastrointestinal tract. It may cause cardiac failure and death.[1]
See also
- Potassium bicarbonate
- Potassium hydrogenacetylenedicarboxylate
References
- ^ a b ChemicalBook (2007) Potassium binoxalate Product Description
- ^ Mark Dugan (2009) Potassium binoxalate product data sheet. Hummel Croton Inc.
- ^ H. Koppers (1973), 'The Elastic Constants of Monoclinic Potassium Hydrogen Oxalate Acta Crystallographica,volume A29, p. 415.
Categories:- Potassium compounds
- Oxalates
Wikimedia Foundation. 2010.
