- Metallo-beta-lactamase protein fold
-
Metallo-beta-lactamase superfamily 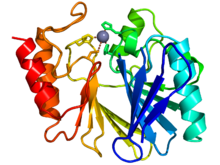
Cartoon diagram of the crystallographic structure of a zinc metallo-beta-lactamase from bacillus cereus (N-terminus = blue, C-terminus = blue) The catalytic zinc ion is depicted as a grey sphere [1]. Identifiers Symbol Lactamase_B Pfam PF00753 InterPro IPR001279 SCOP 1bmc Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary The metallo-beta-lactamase protein fold is a protein domain contained in class B beta-lactamases and a number of other proteins [1]. These proteins include thiolesterases, members of the glyoxalase II family, that catalyse the hydrolysis of S-D-lactoyl-glutathione to form glutathione and D-lactic acid and a competence protein that is essential for natural transformation in Neisseria gonorrhoeae and could be a transporter involved in DNA uptake. Except for the competence protein these proteins bind two zinc ions per molecule as cofactor.
Metallo-beta-lactamases are important enzymes because they are involved in the breakdown of antibiotics by antibiotic-resistant bacteria[2].
See also
- New Delhi metallo-beta-lactamase
References
- ^ a b PDB 1bmc ; Carfi A, Pares S, Duée E, Galleni M, Duez C, Frère JM, Dideberg O (October 1995). "The 3-D structure of a zinc metallo-beta-lactamase from Bacillus cereus reveals a new type of protein fold". EMBO J. 14 (20): 4914–21. PMC 394593. PMID 7588620. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=394593.
- ^ "Shaw, Robert W. (Lubbock, TX, US), Kim, Sung-kun (Lubbock, TX, US)" ("November" "2008"). ""Inhibition of metallo-β-lactamase"". http://www.freepatentsonline.com/7456274.html.
This article includes text from the public domain Pfam and InterPro IPR001279
Categories:- Protein domains
Wikimedia Foundation. 2010.
