- 1,3-Bis(diphenylphosphino)propane
-
1,3-Bis(diphenylphosphino)propane 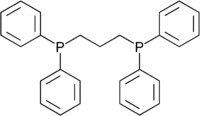
 Propane-1,3-diylbis(diphenylphosphane)
Propane-1,3-diylbis(diphenylphosphane)Identifiers Abbreviations DPPP CAS number 6737-42-4 
PubChem 81219 ChemSpider 73276 
ChEMBL CHEMBL73394 
Jmol-3D images Image 1
Image 2- P(c1ccccc1)(c2ccccc2)CCCP(c3ccccc3)c4ccccc4
c1ccc(cc1)P(CCCP(c2ccccc2)c3ccccc3)c4ccccc4
- InChI=1S/C27H26P2/c1-5-14-24(15-6-1)28(25-16-7-2-8-17-25)22-13-23-29(26-18-9-3-10-19-26)27-20-11-4-12-21-27/h1-12,14-21H,13,22-23H2

Key: LVEYOSJUKRVCCF-UHFFFAOYSA-N
InChI=1/C27H26P2/c1-5-14-24(15-6-1)28(25-16-7-2-8-17-25)22-13-23-29(26-18-9-3-10-19-26)27-20-11-4-12-21-27/h1-12,14-21H,13,22-23H2
Key: LVEYOSJUKRVCCF-UHFFFAOYAP
Properties Molecular formula C27H26P2 Molar mass 412.44 g mol−1 Appearance white solid Solubility in water chlorocarbons  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1,3-Bis(diphenylphosphino)propane (dppp) is a diphosphine often found as a ligand in coordination chemistry.
The diphosphine can be prepared by the reaction of lithium diphenylphosphide and 1,3-dichloropropane.
- 2 Ph2PLi + C3H6Cl2 → Ph2PC3H6PPh2 + 2 LiCl
The diphosphine is a precursor to the complex dichloro(1,3-bis(diphenylphosphino)propane)nickel, which is prepared by combining equimolar portions of the ligand and nickel(II) chloride hexahydrate. This nickel complex serves as a catalyst for the Kumada coupling reaction.[1] Dppp is also used as a ligand for palladium(II) catalysts to co-polymerize carbon monoxide and ethylene to give polyketones.[2]
References
- ^ Kumada, Makota; Tamao, Kohei; Sumitani, Koji (1988), "Phosphine-Nickel Complex Catalyzed Cross-Coupling of Grignard Reagents with Aryl and Alkenyl Halides: 1,2-dibutylbenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0407; Coll. Vol. 6: 407
- ^ Drent, E.; Mul, W. P.; Smaardijk, A. A. (2001). "Polyketones". Encyclopedia Of Polymer Science and Technology. doi:10.1002/0471440264.pst273.
Categories:- Bisphosphanes
- P(c1ccccc1)(c2ccccc2)CCCP(c3ccccc3)c4ccccc4
Wikimedia Foundation. 2010.
