- Dendralene
-
A dendralene is a discrete acyclic cross-conjugated polyene. [1] [2] The simplest dendralene is buta-1,3-diene (1) or [2]dendralene followed by [3]dendralene (2), [4]dendralene (3) and [5]dendralene (4) and so forth. [2]dendralene (butadiene) is the only one not cross-conjugated.
The name dendralene is pulled together from the words dendrimer, linear and alkene. The higher dendralenes are of scientific interest because they open up a large array of new organic compounds from a relatively simple precursor especially by Diels-Alder chemistry. Their cyclic counterparts are aptly called radialenes.
Contents
Synthesis
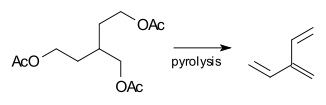
Vinylbutadiene ([3]dendralene) was first prepared in 1955 by pyrolysis of a triacetate: [3] [4]
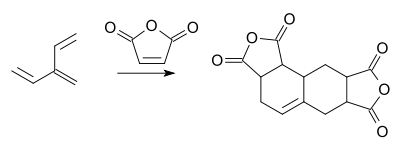
This compound reacts with two equivalents of maleic anhydride in a tandem DA reaction: [5]
With benzoquinone the reaction product was a linear polymer.
Several syntheses of substituted [3]dendralenes have been reported, one via an allene [6] , one via a Horner–Wadsworth–Emmons reaction [7] , one via a cross-coupling reaction [8] and one from an allylic carbonate [9].
One synthetic route to [4]dendralene starts from chloroprene.[10] This compound is converted to a Grignard reagent by action of magnesium metal which is then reacted with copper(I) chloride to an organocopper intermediate which is in turn dimerized using copper(II) chloride in an oxidative coupling reaction to give the butadiene dimer called [4]dendralene.
The [8]-dendralene compound was reported in 2009 [11]:
in a successive Kumada–Tamao–Corriu coupling and Negishi coupling.
Properties
Even-membered dendralenes (e.g. [6]dendralene, [8]dendralene) tend to behave as chains of decoupled and isolated diene units. The ultraviolet absorption maxima equal that of butadiene itself. The dendralenes with an odd number of alkene units are more reactive due to the presence of favorable s-cis diene conformations and Diels-Alder reactions take place more easily with a preference for the termini.
Reactions
With simple dienophiles , dendrales can give quick access to complex molecules in Diels-Alder reactions. Several reaction schemes have been reported [10] [12] [13] [14]
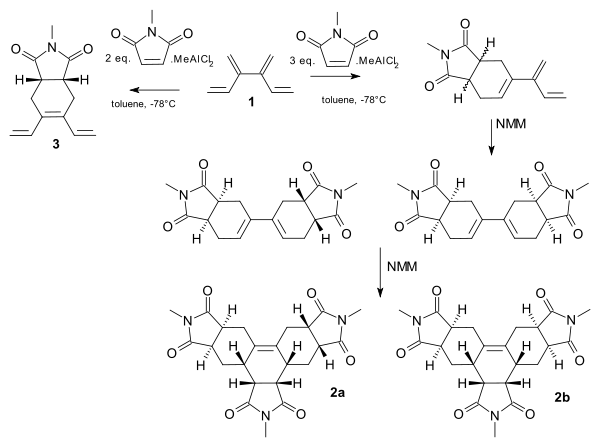
[4]dendralene shows a tandem Diels-Alder reaction with the dienophile N-methyl-maleimide (NMM) [10]. Complete site selectivity is possible with the addition of the Lewis acid methyldichloroaluminium. With one set of premixing and 2 equivalents of NMM, the central diene group is targeted to the monoadduct 3. With another set and a larger amount of dienophile, the terminal groups react and the reaction proceeds from the monoadduct to the trisadducts 2 and 2b.
One reaction variation is cyclopropanation to a compound class called ivyanes with a reported synthesis in a Simmons–Smith reaction (diethyl zinc / trifluoroacetic acid) of the first 6 members. [15]. These 1,1-oligocyclopropanes are stable (except when exposed to acids) and have a large heat of combustion with [6]ivyane exceeding that of cubane. The oligocyclopropane chains adopt a helical conformation.
References
- ^ Henning Hopf, Classics in Hydrocarbon Chemistry, Wiley VCH, 2000.
- ^ Hopf, H. (1984), The Dendralenes—a Neglected Group of Highly Unsaturated Hydrocarbons. Angewandte Chemie International Edition in English, 23: 948–960. doi:10.1002/anie.198409481
- ^ Pyrolysis of Esters. III. Synthesis of 2-Vinylbutadiene, William J. Bailey, James Economy Journal of the American Chemical Society 1955 77 (5), 1133-1136 doi:10.1021/ja01610a014
- ^ 2-Vinyl-1,3-butadiene A. T. Blomquist, Joseph A. Verdol J. Am. Chem. Soc., 1955, 77 (1), pp 81–83 doi:10.1021/ja01606a025
- ^ Polymers. IV. Polymeric Diels-Alder Reactions William J. Bailey, James Economy, Mathew E. Hermes J. Org. Chem., 1962, 27 (9), pp 3295–3299 doi:10.1021/jo01056a074
- ^ Synthesis of cross-conjugated trienes by dimerization of allenes with palladium-phenol catalyst Mieko Arisawa, Takumichi Sugihara and Masahiko Yamaguchi Chem. Commun., 1998, 2615-2616 doi:10.1039/A807527A
- ^ Synthesis of substituted [3]dendralenes and their unique cycloaddition reactions Rekha Singh and Sunil K. Ghosh Chem. Commun., 2011, Advance Article doi:10.1039/C1CC14211A
- ^ Cross-Coupling for Cross-Conjugation: Practical Synthesis and Diels−Alder Reactions of [3]Dendralenes Tanya A. Bradford,, Alan D. Payne,, Anthony C. Willis,,, Michael N. Paddon-Row,, and, Michael S. Sherburn, Organic Letters 2007 9 (23), 4861-4864 doi:10.1021/ol7021998
- ^ Efficient ruthenium-catalyzed synthesis of [3]dendralenes from 1,3-dienic allylic carbonates Kassem Beydoun, Hui-Jun Zhang, Basker Sundararaju, Bernard Demerseman, Mathieu Achard, Zhenfeng Xi and Christian Bruneau Chem. Commun., 2009, 6580-6582 doi:10.1039/B913595B
- ^ a b c Alan D. Payne, Anthony C. Willis, and Michael S. Sherburn (2005). "Practical Synthesis and Diels-Alder Chemistry of [4]Dendralene". Journal of the American Chemical Society 127 (35): 12188–12189. doi:10.1021/ja053772. PMID 16131173.
- ^ Practical Synthesis of the Dendralene Family Reveals Alternation in Behavior Alan D. Payne, Gomotsang Bojase, Michael N. Paddon-Row, and Michael S. Sherburn Angew. Chem. Int. Ed. 2009, 48, doi:10.1002/anie.200901733
- ^ Consecutive Rh(I)-catalyzed Alder-ene/Diels–Alder/Diels–Alder reaction sequence affording rapid entry to polycyclic compounds Tetrahedron, Volume 61, Issue 26, 27 June 2005, Pages 6180-6185 Kay M. Brummond, Lingfeng You doi:10.1016/j.tet.2005.03.141
- ^ Hopf, H. and Yildizhan, Ş. (2011), Highly Functionalized, Angularly Anellated Aromatic Compounds from Dendralenes. European Journal of Organic Chemistry, 2011: 2029–2034. doi:10.1002/ejoc.201001536
- ^ A novel and facile stereocontrolled synthetic method for polyhydro-quinolines and pyridopyridazines via a diene-transmissive Diels–Alder reaction involving inverse electron-demand hetero Diels–Alder cycloaddition of cross-conjugated azatrienes Tetrahedron, Volume 64, Issue 41, 6 October 2008, Pages 9705-9716 Satoru Kobayashi, Tomoki Furuya, Takashi Otani and Takao Saito doi:doi:10.1016/j.tet.2008.07.102
- ^ Synthesis and properties of the ivyanes: the parent 1,1-oligocyclopropanes Gomotsang Bojase, Thanh V. Nguyen, Alan D. Payne, Anthony C. Willis and Michael S. Sherburn Chem. Sci., 2011, 2, 229-232 doi:10.1039/C0SC00500B
Categories:- Alkenes
Wikimedia Foundation. 2010.

![[4]dendralene synthetic scheme from chloroprene](/pictures/enwiki/53/569px-4-dendralene.svg.png)
![[8]-dendralene synthesis](/pictures/enwiki/52/411px-8-dendralene.svg.png)