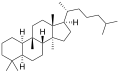
- Cucurbitane
-
Cucurbitane 
Identifiers CAS number 65441-59-0 (5α/β) 
Jmol-3D images
Image 3- C[C@H](CCCC(C)C)[C@@]1([H])CC[C@@]2(C)[C@]3([H])CC[C@@]4([H])C(C)(C)CCC[C@@]4([H])[C@]3(C)CC[C@@]21C (5α)
C[C@H](CCCC(C)C)[C@@]1([H])CC[C@@]2(C)[C@]3([H])CC[C@]4([H])C(C)(C)CCC[C@@]4([H])[C@]3(C)CC[C@@]21C (5β)
Properties Molecular formula C30H54 Molar mass 414.75 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cucurbitane is a chemical compound with formula C30H54 (CAS number 65441-59-0). It is a polycyclic hydrocarbon, specifically a triterpene. It is an isomer of lanostane (specifically 19(10→9β)abeo-lanostane), from which it differs by the formal shift of a methyl group (carbon number 19) from the 10 to the 9β position in the standard steroid numbering scheme.[1][2]
The name is applied to two stereoisomers, distinguished by the prefixes 5α- and 5β, which differ by the handedness of the bonds at a particular carbon atom (number 5 in the standard steroid numbering scheme).[1]
Contents
Derivatives
Natural compounds
Compounds with the basic cucurbitane skeleton are found in many plants, and some are important phytopharmaceuticals,[3] Natural cucurbitane-related compounds include:
Named
- Balsaminapentaol, from Momordica balsamina.[4]
- Balsaminol A, from Momordica balsamina.[4]
- Balsaminol B, from Momordica balsamina.[4]
- Brydioside A from Bryonia dioica [3]:64
- Bryoamaride and derivatives from Bryonia dioica [3]:65,66
- Charantin or foetidin, from Momordica charantia[5] and Momordica foetida[6]
- Charantosides I-VIII, from Momordica charantia.[7]
- Cucurbalsaminol B, from Momordica balsamina.[4]
- Cucurbalsaminol A, from Momordica balsamina.[4]
- Cucurbitacins A-L, O-T [3][8] [9]:3–8
- Datiscosides, from Datisca glomerata [3]:16–19
- Endecaphyllacins A and B, from roots of Hemsleya endecaphylla [9]:1,2
- Hemslecins A and B, from roots of H. endecaphylla [9]
- Lepidolide, from the mushroom Russula lepida [10]
- Karavilagenin E, from Momordica balsamina.[4]
- Khekadaengosides A, B, D and K, from Trichosanthes tricuspidata [3]:57,58,67,68
- Kuguacins A-S, from stems and leaves of Momordica charantia[11][12]
- Kuguaglycosides A-H, from the root of Momordica charantia[13]
- Mogrosides I-V, from the fruits of Siraitia grosvenorii [14]
- Momordicin I, II and 28, from Momordica charantia[15][16]
- Momordicines II and IV, from leaves of Momordica charantia [17]
- Momordicosides A-S, from Momordica charantia fruits [7][18][19]
- Neokuguaglucoside, from Momordica charantia fruits [20]
- Neomogroside, from the fruit of Siraitia grosvenorii.[21]
- Pentanorcucurbitacins A and B [22]:1,2
- Perseapicroside A, from Persea mexicana [3]:44
- Scandenoside R9, from Hemsleya panacis-scandens [3]:45
- Spinosides A and B, from Desfontainia spinosa [3]:61,62
Unnamed
- 3β,7β,23ξ-trihydroxycucurbita-5,24-dien-19-al, soluble in chloroform, melts at 123-125°C, from Momordica charantia, Momordica foetida.[23]:1
- 3β,7β,25-trihydroxycucurbita-5,23-dien-19-al, soluble in chloroform, melts at 188-191°C, from Momordica charantia, Momordica foetida[23]:2
- 3β,7β-dihydroxy-25-methoxycucurbita-5,23-dien-19-al, soluble in chloroform, from Momordica charantia, Momordica foetida[23]:3
- 5β,19-epoxy-25-methoxycucurbita-6,23-dien-3β,19-diol, soluble in chloroform, melts at 182-184°C, from Momordica foetida[23]:4
- 5β,19-epoxycucurbita-6,23-dien-3β,19,25-triol, soluble in chloroform, from Momordica foetida[23]:5
- 5β,19-epoxy-19-methoxycucurbita-6,23-dien-3β,25-diol, soluble in chloroform, melts at 102-104°C, from Momordica charantia, Momordica foetida[23]:6
- 5β,19-epoxy-19,25-dimethoxycucurbita-6,23-dien-3β-ol, soluble in chloroform, from Momordica charantia, Momordica foetida[23]:7
- 5β,19-epoxy-25-methoxycucurbita-6,23-dien-3β-ol, soluble in chloroform, melts at 139-141°C, from Momordica charantia, Momordica foetida[23]:8
- 2,16-dihydroxy-22,23,24,25,26,27-hexanorcucurbit-5-en-11,20-dione 2-O-β-D-glucopyranoside, soluble in ethanol, from Cucurbita pepo fruits (25 mg/15 kg) [8]:3
- 16-hydroxy-22,23,24,25,26,27-hexanorcucurbit-5-en-11,20-dione 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside, white powder, soluble in ethanol, from Cucurbita pepo fruits (12 mg/15 kg)[8]:4
- 7-methoxycucurbita-5,24-diene-3β,23(R)-diol, from Momordica balsamina [24]
- 25,26,27-trinorcucurbit-5-ene-3,7,23-trione C27H40O3, white powder, soluble in methanol, from stems of Momordica charantia (6 mg/18 kg)[22]:3
See also
- Goyaglicoside
- Karaviloside
- Momordenol, from Momordica charantia[15]
- edit] References
- ^ a b IUPAC Commission on the Nomenclature of Organic Chemistry and IUPAC-IUB Commission on Biochemical Nomenclature (1969), The Nomenclature of Steroids — Revised Tentative Rules. European J. of Biochemistry, volume 10, 1-19
- ^ Satish Kumar and Raj Kumar (1991), Dictionary of Biochemistry. Anmol Publications, India
- ^ a b c d e f g h i Jian Chao Chen, Ming Hua Chiu, Rui Lin Nie, Geoffrey A. Cordell and Samuel X. Qiu (2005), "Cucurbitacins and cucurbitane glycosides: structures and biological activities" Natural Product Reports, volume 22, pages 386-399 doi:10.1039/B418841C
- ^ a b c d e f Cátia Ramalhete,Tayyab A. Mansoor, Silva Mulhovo, Joseph Molnár, and Maria-José U. Ferreira (2009), "Cucurbitane-Type Triterpenoids from the African Plant Momordica balsamina." Journal of Natural Products, volume 72, pages 2009–2013. doi:10.1021/np900457u
- ^ M. M. Lolitkar and M. R. Rajarama Rao (1962), "Note on a Hypoglycaemic Principle Isolated from the fruits of Momordica charantia". Journal of the University of Bombay, volume 29, pages 223-224
- ^ A. A. Olaniyi (1975), "A Neutral Constituent of Momordica foetida". Lloydia, volume 38, issue 4, pages 361-362. School of Pharmacy, University of Ife, Ile-Ife, Nigeria PubMed
- ^ a b Toshihiro Akihisa, Naoki Higo, Harukuni Tokuda, Motohiko Ukiya, Hiroyuki Akazawa, Yuichi Tochigi, Yumiko Kimura, Takashi Suzuki, and Hoyoku Nishino (2007), "Cucurbitane-Type Triterpenoids from the Fruits of Momordica charantia and Their Cancer Chemopreventive Effects". Journal of Natural Products, volume 70, pages 1233-1239. doi:10.1021/np068075p
- ^ a b c Da-Cheng Wang, Hong-Yu Pan, Xu-Ming Deng, Hua Xiang, Hui-Yuan Gao, Hui Cai, and Li-Jun Wu (2007), "Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua". Journal of Asian Natural Products Research, volume 9, issue 6, pages 525–529.
- ^ a b c Jian-Chao Chen, Gao-Hong Zhang, Zhong-Quan Zhang, Ming-Hua Qiu, Yong-Tang Zheng, Liu-Meng Yang, Kai-Bei Yu (2008), "Octanorcucurbitane and Cucurbitane Triterpenoids from the Tubers of Hemsleya endecaphylla with HIV-1 Inhibitory Activity". J. Nat. Prod. volume 71, pages 153–155 doi:10.1021/np0704396
- ^ Jian-Wen Tan, Ze-Jun Dong, Zhi-Hui Ding and Ji-Kai Liu (2002), "Lepidolide, a Novel Seco-ring-A Cucurbitane Triterpenoid from Russula lepida (Basidiomycetes)". Zeitschrift für Naturforschung Series C, volume 57C issue 11/12, pages 963-965.
- ^ Jian-Chao Chen, Wu-Qing Liu, Lu Lu, Ming-Hua Qiu, Yong-Tang Zheng, Liu-Meng Yang, Xian-Min Zhang, Lin Zhou and Zhong-Rong Li (2009), "Kuguacins F–S, cucurbitane triterpenoids from Momordica charantia". Phytochemistry, volume 70, issue 1, pages 133-140 doi:10.1016/j.phytochem.2008.10.011
- ^ J. C. Chen, R. R. Tian, M. H. Qiu, L. Lu, Y. T. Zheng, Z. Q. Zhang (2008), "Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia." Phytochemistry, volume 69, pages 1043–1048
- ^ Jian-Chao Chen, Lu Lu, Xian-Ming Zhang, Lin Zhou, Zhong-Rong Li, and Ming-Hua Qiu (2008), "Eight New Cucurbitane Glycosides, Kuguaglycosides A–H, from the Root of Momordica charantia L.". Helvetica Chimica Acta, volume 91, issue 5, pages 920-928. doi:10.1002/hlca.200890097
- ^ Midori Takasaki, Takao Konoshima, Yuji Murata, Masaki Sugiura, Hoyoku Nishino, Harukuni Tokuda, Kazuhiro Matsumoto, Ryoji Kasai, and Kazuo Yamasaki (2003) "Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori". Cancer Letters, volume 198, pages 37–42
- ^ a b Sabira Begum, Mansour Ahmed, Bina S. Siddiqui, Abdullah Khan, Zafar S. Saify, and Mohammed Arif (1997), "Triterpenes, A Sterol and a Monocyclic Alcohol From Momordica Charantia." Phytochemistry, volume 44, issue 7, pages 1313-1320
- ^ Majekodunmi Fatope, Yoshio Takeda, Hiroyasu Yamashita, Hikaru Okabe, and Tatsuo Yamauchi (1990), "New cucurbitane trirterpenoids from Momordica charantia." Journal of Natural Products, volume 53, issue 6, pages 1491-1497.
- ^ Daniel Bisrat Mekuria, Takehiro Kashiwagi, Shin-ichi Tebayashi, and Chul-Sa Kim (2006)"Cucurbitane Glucosides from Momordica charantia Leaves as Oviposition Deterrents to the Leafminer, Liriomyza trifolii". Z. Naturforsch., volume 61c, pages 81–86
- ^ a b c d e Jie-Qing Liu, Jian-Chao Chen, Cui-Fang Wang and Ming-Hua Qiu (2009), "New Cucurbitane Triterpenoids and Steroidal Glycoside from Momordica charantia". Molecules, volume 14, pages 4804-4813 doi:10.3390/molecules14124804
- ^ Liva Harinantenaina, Michi Tanaka, Shigeru Takaoka, Munehiro Oda, Orie Mogami, Masayuki Uchida, and Yoshinori Asakawa (2006), "Momordica charantia Constituents and Antidiabetic Screening of the Isolated Major Compounds". Chem. Pharm. Bull. volume 54, issue 7, pages 1017—1021. doi:10.1248/cpb.54.1017
- ^ Jie‐Qing Liu, Jian‐Chao Chen, Cui‐Fang Wang and Ming‐Hua Qiu (2010), "One new cucurbitane triterpenoid from the fruits of Momordica charantia". European Journal Chemistry, volume 1, issue 4, pages 294‐296 doi:10.5155/eurjchem.1.4.294‐296.131
- ^ Si Jian-yong, Chen Di-hua, Chang Qi and Shen Lian-gang (1996), Isolation and Determination of Cucurbitane-Glycosides from Fresh Fruits of Siraitia Grosvenorii. Journal of Integrative Plant Biology, volume 38, issue 6, pages, 489–494
- ^ a b Chiy-Rong Chen, Yun-Wen Liao, Lai Wang, Yueh-Hsiung Kuo, Hung-Jen Liu, Wen-Ling Shih, Hsueh-Ling Cheng and Chi-I Chang (2010). "Cucurbitane Triterpenoids from Momordica charantia and Their Cytoprotective Activity in tert-Butyl Hydroperoxide-Induced Hepatotoxicity of HepG2 Cells". Chemical & pharmaceutical bulletin, volume 58, issue 12, pages 1639-1642. doi:10.1248/cpb.58.1639
- ^ a b c d e f g h Dulcie A. Mulholland, Vikash Sewram, Roy Osborne, Karl H. Pegel and Joseph D. Connolly (1997), "Cucurbitane triterpenoids from the leaves of Momordica foetida." Phytochemistry, volume 45, issue 2, pages 391-395. doi:10.1016/S0031-9422(96)00814-X
- ^ Gabriella Spengler, Cátia Ramalhete, Marta Martins, Ana Martins, Julianna Serly, Miguel Viveiros, Joseph Molnár, Noélia Duarte, Silva Mulhovos, Maria-José U. Ferreira, Leonard Amaral (2010), "Evaluation of Cucurbitane-type Triterpenoids from Momordica balsamina on P-Glycoprotein (ABCB1) by Flow Cytometry and Real-time Fluorometry", Anticancer Research, volume 30, pages 4867-4871
Categories:- Triterpenes
- C[C@H](CCCC(C)C)[C@@]1([H])CC[C@@]2(C)[C@]3([H])CC[C@@]4([H])C(C)(C)CCC[C@@]4([H])[C@]3(C)CC[C@@]21C (5α)
Wikimedia Foundation. 2010.