- Costunolide
-
Costunolide  (3aS,6E,10E,11aR)-6,10-dimethyl-3-methylene-3,3a,4,5,8,9-hexahydrocyclodeca[b]furan-2(11aH)-one
(3aS,6E,10E,11aR)-6,10-dimethyl-3-methylene-3,3a,4,5,8,9-hexahydrocyclodeca[b]furan-2(11aH)-oneIdentifiers CAS number 553-21-9 
PubChem 5281437 ChemSpider 4444782 
UNII 4IK578SA7Z 
MeSH (+)-Costunolide ChEBI CHEBI:3900 
ChEMBL CHEMBL205612 
Jmol-3D images Image 1 - O=C/1O[C@@H]2/C=C(/CC/C=C(/CC[C@H]2C\1=C)C)C
Properties Molecular formula C15H20O2 Molar mass 232.318  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
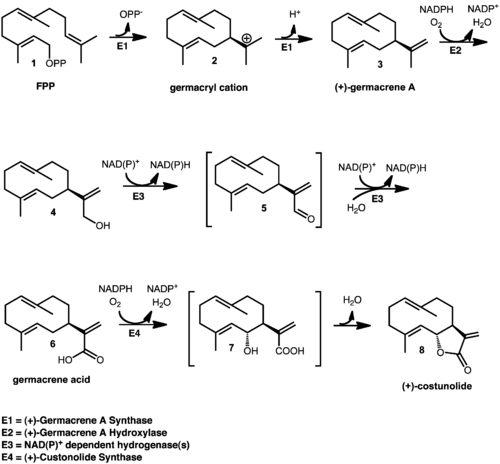
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references (+)-Costunolide is a sesquiterpene lactone that is synthesized through the mevalonate pathway, seen in Figure 1. The synthesis begins with the cyclization of compound 1 , farnesyl pyrophosphate (FPP), which is mediated by a sesquiterpene cyclase, (+)-germacrene A synthase, to form compound 2, (+)-germacryl cation.[1] Inside this same enzyme, a proton is lost to form 3, (+)-germacrene A.[2] The isoprenyl side chain of (+)-germacrene A is then hydroxylated by (+)-germacrene A hydroxylase, which is a cytochrome P450 enzyme, to form 4.[1] NAD(P)+ dependent hydrogenase(s) then oxidize 4, germacra-1(10),4,11(13)-trien-12-ol, through the intermediate 5, germacra-1(10),4,11(13)-trien-12-al to form compound 6, germacrene acid. The cyctochrome P450 enzyme, (+)-costunolide synthase, which is a NADPH and O2 dependent enzyme, then oxidizes germacrene acid to give the alcohol intermediate, 7, which then cyclizes to form the lactone 8, (+)-costunolide.[3]
Figure 1. Biosynthesis of (+)-Costunolide.[2]
References
- ^ a b Kraker, J.; Franssen, M.; Dalm, M.; Groot, A.; Bouwmeester, H. (April 2001). "Biosynthesis of Germacrene A Carboxylic Acid in Chicory Roots. Demonstration of a Cytochrome P450 (+)-Germacrene A Hydroxylase and NADP+-Dependent Sesquiterpenoid Dehydrogenase(s) Involved in Sesquiterpene Lactone Biosynthesis". Plant Physiology 125 (4): 1930–1940. doi:10.1104/pp.125.4.1930. PMC 88848. PMID 11299372. http://www.plantphysiol.org/cgi/content/full/125/4/1930?maxtoshow=&hits=10&RESULTFORMAT=&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&volume=125&firstpage=1930&resourcetype=HWCIT.
- ^ a b Dewick, Paul M. (2009). Medicinal Natural Products: A Biosynthetic Approach. West Sussex, United Kingdom: John Wiley & Sons Ltd. p. 214.
- ^ Kraker, J.; Franssen, M.; Joerink, M.; Groot, A.; Bouwmeester, H. (April 2002). "Biosynthesis of Costunolide, Dihydrocostunolide, and Leucodin. Demonstration of Cytochrome P450-Catalyzed Formation of the Lactone Ring Present in Sesquiterpene Lactones of Chicory". Plant Physiology 129: 257–258. doi:10.1104/pp.010957. http://www.plantphysiol.org/cgi/content/full/125/4/1930?maxtoshow=&hits=10&RESULTFORMAT=&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&volume=125&firstpage=1930&resourcetype=HWCIT.
Categories:- Sesquiterpene lactones
Wikimedia Foundation. 2010.