- Polyamino carboxylic acid
-
A polyamino carboxylic acid (complexone) is a compound containing one or more nitrogen atoms connected through carbon atoms to one or more carboxyl groups. Polyamino carboxylates, which have lost acidic protons, form strong complexes with metal ions by donation of electron pairs from the nitrogen and oxygen atoms to the metal ion to form multiple chelate rings. This property makes polyamino carboxylic acids useful in a wide variety of chemical, medical and environmental applications.[1]
Structure
The simplest substance to which this class of compounds can be related is glycine, H2NCH2CO2H, in which the amino group, NH2,is separated from the carboxyl group, COOH by a single methylene group, CH2. When the carboxyl group is deprotonated the glycinate ion can function as a bidentate ligand, donating through the nitrogen atom and a carboxylate oxygen atom, to form chelate complexes of metal ions.[2]
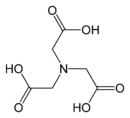
Replacement of a hydrogen atom on the nitrogen of glycine by another acetate residue, –CH2CO2H gives iminodiacetic acid, IDA, which is a tridentate ligand. Further substitution gives nitrilotriacetic acid, NTA, which is a tetradentate ligand.[3] These compounds can be described as polyamino acetates. Starting from β-alanine a series of polyamino propionates can be envisaged.
Higher denticity is achieved by linking two or more units together. EDTA contains two IDA units with the nitrogen atoms linked by two methylene groups and is hexadentate. DTPA has two CH2CH2 bridges linking three nitrogen atoms and is octadentate. TTHA[1] has ten potential donor atoms.
Applications
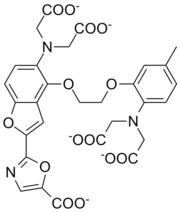
The chelating properties of polyamino carboxylates can be engineered by varying the groups linking the nitrogen atoms so as to increase selectivity for a particular metal ion. The number of carbon atoms between the nitrogen and carboxyl group can also be varied and substituents can be placed on these carbon atoms. Altogether this allows for a vast range of possibilities. Fura-2 is noteworthy as it combines two functionalities: it has high selectivity for calcium over magnesium and it has a substituent which makes the complex fluorescent when it binds calcium. This provides a means of determining the calcium content in intra-cellular fluid. Details concerning applications of the following examples can be found in the individual articles and/or reference.
Fura-2 IDA[1] NTA[3] 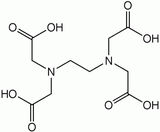
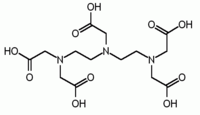
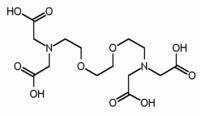
EDTA DTPA[1] EGTA 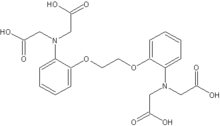
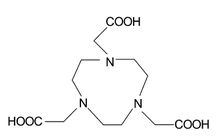
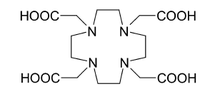
BAPTA NOTA[1] DOTA[1] References
- ^ a b c d e f Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. (2005). "Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications* (IUPAC Technical Report)". Pure Appl. Chem., 77 (8): 1445–1495. doi:10.1351/pac200577081445. pdf
- ^ Schwarzenbach, G (1952). "Der Chelateffekt". Helv. Chim. Acta 35: 2344–2359. doi:10.1002/hlca.19520350721.
- ^ a b Anderegg, G (1982). "Critical survey of stability constants of NTA complexes". Pure Appl. Chem., 54 (12): 2693–2758. doi:10.1351/pac198254122693. pdf
Categories:- Equilibrium chemistry
- Chelating agents
Wikimedia Foundation. 2010.