- Aromadedrin
-
Aromadedrin 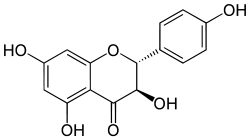 Aromadendrin
Aromadendrin
(2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-oneOther namesDihydrokaempferol
Aromadendrol
(+)-aromadendrin
(+)-DihydrokaempferolIdentifiers CAS number 480-20-6 
PubChem 122850 ChemSpider 109514 
ChEBI CHEBI:15401 
ChEMBL CHEMBL9323 
Jmol-3D images Image 1 - C1=CC(=CC=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O
Properties Molecular formula C15H12O6 Molar mass 288.25 g/mol Exact mass 288.063388  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Aromadedrin (dihydrokaempferol) is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.[1]
Contents
Metabolism
The enzyme dihydrokaempferol 4-reductase uses cis-3,4-leucopelargonidin and NADP+ to produce (+)-aromadedrin, NADPH, and H+.
Glycosides
(2R,3R)-trans-Aromadendrin-7-O-beta-D-glucopyranoside-6"-(4"-hydroxy-2"-methylene butanoate) is an acetylated glucoside of aromadendrin isolated from the stem bark of Afzelia bella[2] (Fabaceae).
Phellamurin is the 8-prenyl 7-glucoside derivative of aromadendrin.
Chemistry
(+)-Leucopelargonidin, (2R,3S,4R)-3,4,5,7,4-pentahydroxyflavan, can be synthesized from (+)-aromadendrin by sodium borohydride reduction.[3]
References
- ^ Aromadendrin, apigenin, and kaempferol from the wood of Pinus sibirica, V. I. Lutskii, A. S. Gromova and N. A. Tyukavkina, 1971
- ^ Constituents of Afzelia bella stem bark. Binutu OA, Cordell GA.
- ^ Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br. Werner Heller, Lothar Britsch, Gert Forkmann and Hans Grisebach, 1984
3-Hydroxyflavanones: Ampelopsin (Dihydromyricetin) | Aromadedrin (Dihydrokaempferol) | Dihydrogossypetin | Dihydromorin | Fustin (Dihydrofisetin) | Garbanzol | Taxifolin (Dihydroquercetin)O-methylated flavanonols dihydroflavonol 3-O-glycosides Lecontin | (+)-fustin glucosideGlycosides Acetylated glycosides PhellamurinThis article about a natural phenol is a stub. You can help Wikipedia by expanding it.
