- Thulium(III) chloride
-
Thulium(III) chloride 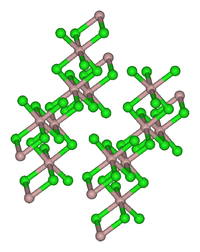 Thulium(III) chlorideOther namesThulium chloride, thulium trichloride
Thulium(III) chlorideOther namesThulium chloride, thulium trichlorideIdentifiers CAS number 13537-18-3 PubChem 24867527 RTECS number XP0525000 Properties Molecular formula TmCl3 Molar mass 275.292 g/mol Appearance yellow crystals Density 3.98 g/cm³ Melting point 824°C
Boiling point 1490°C[1]
Solubility in water heptahydrate: very soluble Solubility heptahydrate: very soluble in ethanol[2] Structure Crystal structure Monoclinic, mS16 Space group C12/m1, No. 12 Coordination
geometry6[1] Thermochemistry Std enthalpy of
formation ΔfHo298966.6 kJ/mol[3] Hazards S-phrases S26, S36[4] Main hazards Xi (Irritant) Related compounds Other anions Thulium(III) oxide Other cations Erbium(III) chloride
Ytterbium(III) chloride
Thulium(II) chloride chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Thulium(III) chloride or thulium trichloride is the chemical compound composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions.[5]
Reactions
The hydrated form of thulium(III) chloride can be obtained by adding thulium(III) oxide to concentrated hydrochloric acid[2]. Thulium(III) chloride reacts with strong bases to make thulium(III) oxide.
References
- ^ a b "Chemistry: Periodic Table: Thulium: compound data (thulium (III) chloride)". WebElements. http://202.114.88.54/g/web18/wangluo/webelements/webelements/compounds/text/tm/cl3tm1-13537183.html. Retrieved 2008-06-27.
- ^ a b Spencer, James F. (1919). The Metals of the Rare Earths. New York: Longmans, Green, and Co. pp. 152. http://books.google.com/?id=W2zxN_FLQm8C&pg=PA152. Retrieved 2008-06-27
- ^ Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 512. ISBN 0849386713. http://books.google.com/?id=kTnxSi2B2FcC&pg=PT1017. Retrieved 2008-06-27
- ^ "439649 Thulium(III) chloride anhydrous, powder, 99.99% trace metals basis". Sigma-Aldrich. https://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/439649. Retrieved 2008-06-27.
- ^ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
Thulium compounds TmCl3 · Tm2O3
Categories:- Inorganic compound stubs
- Thulium compounds
- Chlorides
- Metal halides
Wikimedia Foundation. 2010.
