- Supercritical drying
-
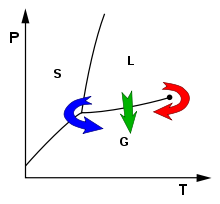 Supercritical drying (red arrow) goes beyond the critical point of the working fluid in order to avoid the direct liquid–gas transition seen in ordinary drying (green arrow) or the two phase changes in freeze drying (blue arrow).
Supercritical drying (red arrow) goes beyond the critical point of the working fluid in order to avoid the direct liquid–gas transition seen in ordinary drying (green arrow) or the two phase changes in freeze drying (blue arrow).
Supercritical drying is a process to remove liquid in a precisely controlled way. It is useful in the production of microelectromechanical systems (MEMS), the drying of spices, the production of aerogel, and in the preparation of biological specimens for scanning electron microscopy.
As the substance in a liquid body crosses the boundary from liquid to gas (see green arrow in phase diagram), the liquid changes into gas at a finite rate, while the liquid body's volume decreases. When this happens within a heterogeneous environment, surface tension in the liquid body pulls against any solid structures the liquid might be in contact with. Delicate structures such as cell walls, the dendrites in silica gel, and the tiny machinery of microelectromechanical devices, tend to be broken apart by this surface tension as the liquid–solid interface moves by.
To avoid this, the sample can be brought via two possible alternate paths from the liquid phase to the gas phase without crossing the liquid–gas boundary on the phase diagram. In freeze-drying, this means going around to the left (low temperature, low pressure; blue arrow). However, some structures are disrupted even by the solid–gas boundary. Supercritical drying, on the other hand, goes around the line to the right, on the high-temperature, high-pressure side (red arrow). This route from liquid to gas does not cross any phase boundary, instead passing through the supercritical region, where the distinction between gas and liquid ceases to apply. Densities of the liquid phase and vapor phase becomes equal at critical point of drying.
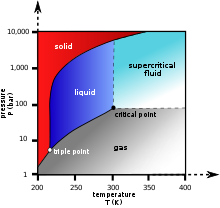
Fluids suitable for supercritical drying include carbon dioxide (critical point 304.25 K at 7.39 MPa or 31.1 °C at 1072 psi) and freon (≈300 K at 3.5–4 MPa or 25–0 °C at 500–600 psi). Nitrous oxide has similar physical behavior to carbon dioxide, but is a powerful oxidizer in its supercritical state. Supercritical water is also a powerful oxidizer, partly because its critical point occurs at such a high temperature (647 K, 374 °C) and pressure (22.064 MPa, 3212 psi).[1]
In most such processes, acetone is first used to wash away all water, exploiting the complete miscibility of these two fluids. The acetone is then washed away with high pressure liquid carbon dioxide, the industry standard now that freon is unavailable. The liquid carbon dioxide is then heated until its temperature goes beyond the critical point, at which time the pressure can be gradually released, allowing the gas to escape and leaving a dried product.
References
External links
Categories:- Industrial processes
- Microtechnology
Wikimedia Foundation. 2010.