- Glyceryl laurate
-
Glyceryl laurate 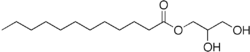 dodecanoic acid 2,3-dihydroxypropyl esterOther namesMonolaurin
dodecanoic acid 2,3-dihydroxypropyl esterOther namesMonolaurin
Monolauroylglycerin
Glycerol monolaurateIdentifiers CAS number 27215-38-9 
PubChem 14871 ChemSpider 14181 
UNII Y98611C087 
ChEMBL CHEMBL510533 
Jmol-3D images Image 1 - O=C(OCC(O)CO)CCCCCCCCCCC
Properties Molecular formula C15H30O4 Molar mass 274.40 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Glyceryl laurate, also glycerol monolaurate or monolaurin, is a monoglyceride surfactant. It is the mono-ester formed from glycerol and lauric acid. Its chemical formula is C15H30O4.
It is most commonly used in deodorants.
In the human system, lauric acid is converted into monolaurin. Monolaurin is found in coconut oil and human breast milk.[1] It may be useful in the prevention and treatment of bacterial infection[2] or generally as a microbicide.[3]
Contents
See also
References
- ^ Hegde, BM (2006). "View Point: Coconut Oil – Ideal Fat next only to Mother's Milk (Scanning Coconut's Horoscope)" (pdf). JIACM 7: 16–19. http://medind.nic.in/jac/t06/i1/jact06i1p16.pdf.
- ^ Preuss, HG; Echard, B; Enig, M; Brook, I; Elliott, TB (2005). "Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria". Molecular and cellular biochemistry 272 (1-2): 29–34. PMID 16010969.
- ^ Isaacs, CE (2001). "The antimicrobial function of milk lipids". Advances in nutritional research 10: 271–85. PMID 11795045.
Further reading
- Lieberman, Shari, Mary G. Enig, Harry G. Preuss. (December 2006). A Review of Monolaurin and Lauric Acid: Natural Virucidal and Bactericidal Agents. Alternative and Complementary Therapies 12 (6): 310-314. doi:10.1089/act.2006.12.310
External links

This article about an organic compound is a stub. You can help Wikipedia by expanding it.
