- Clemmensen reduction
-
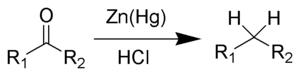
Clemmensen reduction is a chemical reaction described as a reduction of ketones (or aldehydes) to alkanes using zinc amalgam and hydrochloric acid.[1][2][3] This reaction is named after Erik Christian Clemmensen, a Danish chemist.[4]
The Clemmensen reduction is particularly effective at reducing aryl-alkyl ketones.[5][6] With aliphatic or cyclic ketones, zinc metal reduction is much more effective.[7]
The substrate must be stable in the strongly acidic conditions of the Clemmensen reduction. Acid sensitive substrates should be reacted in the Wolff-Kishner reduction, which utilizes strongly basic conditions. As a result of Clemmensen Reduction, the carbon of the carbonyl group involved is converted from sp2 hybridisation to sp3 hybridisation. The oxygen atom is lost in the form of one molecule of water.
References
- ^ Clemmensen, E. (1913). Chemische Berichte 46: 1837.
- ^ Clemmensen, E. (1914). Chemische Berichte 47: 51.
- ^ Clemmensen, E. (1914). Chemische Berichte 47: 681.
- ^ Biographies of Chemists, accessed 6 Feb 2007
- ^ "γ-Phenylbutyric acid", Org. Synth. 2: 499, 1943, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0499; Vol. 15, p.64 (1935)
- ^ "Creosol", Org. Synth. 4: 203, 1963, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4p0203; Vol. 33, p.17 (1953).
- ^ "Modified Clemmensen Reduction: Cholestane", Org. Synth. 6: 289, 1988, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0289; Vol. 53, p.86 (1973).
Reviews
- Martin, E. L. (1942). Org. React. 1: 155.
- Buchanan, J. G. St. C.; Woodgate, P. D. (1969). "The Clemmensen reduction of difunctional ketones". Quart. Rev. 23: 522. doi:10.1039/QR9692300522.
- Vedejs, E. (1975). Org. React. 22: 40.
- Yamamura, S.; Nishiyama, S. (1991). Comp. Org. Syn. 8: 309–313.
See also
- Haworth phenanthrene synthesis
- Wolff-Kishner reduction
Categories:- Organic redox reactions
- Name reactions
Wikimedia Foundation. 2010.