- One-pot synthesis
-
In chemistry a one-pot synthesis is a strategy to improve the efficiency of a chemical reaction whereby a reactant is subjected to successive chemical reactions in just one reactor. This is much desired by chemists because avoiding a lengthy separation process and purification of the intermediate chemical compounds would save time and resources while increasing chemical yield.
An example of a one-pot synthesis is the total synthesis of tropinone or the Gassman indole synthesis. Sequential one-pot syntheses can be used to generate even complex targets with multiple stereocentres, such as oseltamivir,[1] which may significantly shorten the number of steps required overall and have important commercial implications.
A sequential one-pot synthesis with reagents added to a reactor one at a time and without work-up is also called a telescoping synthesis.
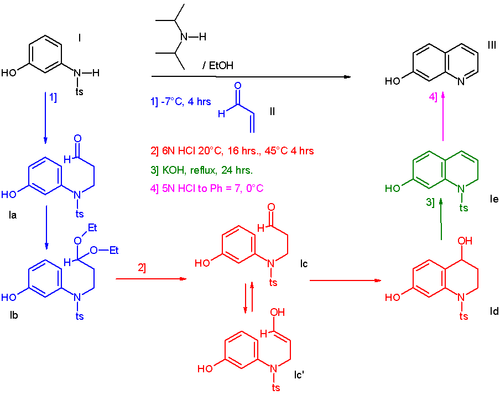
In one such procedure [2] the reaction of 3-N-tosylaminophenol I with acrolein II affords a hydroxyl substituted quinoline III through 4 sequential steps without workup of the intermediate products [3]:
References
- ^ Ishikawa, H.; Suzuki, T.; Hayashi, Y. (2009). "High-yielding synthesis of the anti-influenza neuramidase inhibitor (-)-oseltamivir by three "one-pot" operations". Angewandte Chemie (International ed. in English) 48 (7): 1304–1307. doi:10.1002/anie.200804883. PMID 19123206.
- ^ Cameron, M.; Hoerrner, R. S.; Mcnamara, J. M.; Figus, M.; Thomas, S. (2006). "One-Pot Preparation of 7-Hydroxyquinoline". Organic Process Research & Development 10 (1): 149. doi:10.1021/op0501545.
- ^ The addition of acrolein (blue) is a Michael reaction catalyzed by N,N-diisopropylamine, the presence of ethanol converts the aldehyde group to an acetal but this process is reversed when hydrochloric acid is introduced (red). The enolate reacts as an electrophile in a Friedel-Crafts reaction with ring-closure. The alcohol group is eliminated in presence of potassium hydroxide (green) and when in the final step the reaction medium is neutralized to pH 7 (magenta) the tosyl group is eliminated as well.
Categories:
Wikimedia Foundation. 2010.