- DFFB
-
DNA fragmentation factor 40 kDa 
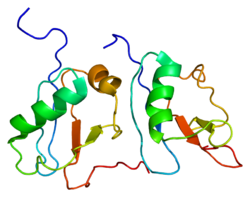
crystal structure of caspase-activated dnase (cad) Identifiers Symbol DFF40 Pfam PF09230 InterPro IPR015311 SCOP 1v0d Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary DNA fragmentation factor subunit beta is a protein that in humans is encoded by the DFFB gene.[1][2][3]
Apoptosis is a cell death process that removes toxic and/or useless cells during mammalian development. The apoptotic process is accompanied by shrinkage and fragmentation of the cells and nuclei and degradation of the chromosomal DNA into nucleosomal units. DNA fragmentation factor (DFF) is a heterodimeric protein of 40-kD (DFFB) and 45-kD (DFFA) subunits. DFFA is the substrate for caspase-3 and triggers DNA fragmentation during apoptosis. DFF becomes activated when DFFA is cleaved by caspase-3. The cleaved fragments of DFFA dissociate from DFFB, the active component of DFF. DFFB has been found to trigger both DNA fragmentation and chromatin condensation during apoptosis. Multiple alternatively spliced transcript variants encoding distinct isoforms have been found for this gene, but the biological validity of some variants has not been determined.[3]
Interactions
DFFB has been shown to interact with DFFA.[4][5]
References
- ^ Liu X, Zou H, Slaughter C, Wang X (May 1997). "DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis". Cell 89 (2): 175–84. doi:10.1016/S0092-8674(00)80197-X. PMID 9108473.
- ^ Halenbeck R, MacDonald H, Roulston A, Chen TT, Conroy L, Williams LT (Aug 1998). "CPAN, a human nuclease regulated by the caspase-sensitive inhibitor DFF45". Curr Biol 8 (9): 537–40. doi:10.1016/S0960-9822(98)79298-X. PMID 9560346.
- ^ a b "Entrez Gene: DFFB DNA fragmentation factor, 40kDa, beta polypeptide (caspase-activated DNase)". http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1677.
- ^ Ewing, Rob M; Chu Peter, Elisma Fred, Li Hongyan, Taylor Paul, Climie Shane, McBroom-Cerajewski Linda, Robinson Mark D, O'Connor Liam, Li Michael, Taylor Rod, Dharsee Moyez, Ho Yuen, Heilbut Adrian, Moore Lynda, Zhang Shudong, Ornatsky Olga, Bukhman Yury V, Ethier Martin, Sheng Yinglun, Vasilescu Julian, Abu-Farha Mohamed, Lambert Jean-Philippe, Duewel Henry S, Stewart Ian I, Kuehl Bonnie, Hogue Kelly, Colwill Karen, Gladwish Katharine, Muskat Brenda, Kinach Robert, Adams Sally-Lin, Moran Michael F, Morin Gregg B, Topaloglou Thodoros, Figeys Daniel (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. (England) 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1847948.
- ^ McCarty, J S; Toh S Y, Li P (Oct. 1999). "Study of DFF45 in its role of chaperone and inhibitor: two independent inhibitory domains of DFF40 nuclease activity". Biochem. Biophys. Res. Commun. (UNITED STATES) 264 (1): 176–80. doi:10.1006/bbrc.1999.1497. ISSN 0006-291X. PMID 10527860.
Further reading
- Enari M, Sakahira H, Yokoyama H, et al. (1998). "A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD". Nature 391 (6662): 43–50. doi:10.1038/34112. PMID 9422506.
- Liu X, Li P, Widlak P, et al. (1998). "The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis". Proc. Natl. Acad. Sci. U.S.A. 95 (15): 8461–6. doi:10.1073/pnas.95.15.8461. PMC 21098. PMID 9671700. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=21098.
- Mukae N, Enari M, Sakahira H, et al. (1998). "Molecular cloning and characterization of human caspase-activated DNase". Proc. Natl. Acad. Sci. U.S.A. 95 (16): 9123–8. doi:10.1073/pnas.95.16.9123. PMC 21302. PMID 9689044. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=21302.
- Gu J, Dong RP, Zhang C, et al. (1999). "Functional interaction of DFF35 and DFF45 with caspase-activated DNA fragmentation nuclease DFF40". J. Biol. Chem. 274 (30): 20759–62. doi:10.1074/jbc.274.30.20759. PMID 10409614.
- McCarty JS, Toh SY, Li P (1999). "Study of DFF45 in its role of chaperone and inhibitor: two independent inhibitory domains of DFF40 nuclease activity". Biochem. Biophys. Res. Commun. 264 (1): 176–80. doi:10.1006/bbrc.1999.1497. PMID 10527860.
- McCarty JS, Toh SY, Li P (1999). "Multiple domains of DFF45 bind synergistically to DFF40: roles of caspase cleavage and sequestration of activator domain of DFF40". Biochem. Biophys. Res. Commun. 264 (1): 181–5. doi:10.1006/bbrc.1999.1498. PMID 10527861.
- Lugovskoy AA, Zhou P, Chou JJ, et al. (2000). "Solution structure of the CIDE-N domain of CIDE-B and a model for CIDE-N/CIDE-N interactions in the DNA fragmentation pathway of apoptosis". Cell 99 (7): 747–55. doi:10.1016/S0092-8674(00)81672-4. PMID 10619428.
- Judson H, van Roy N, Strain L, et al. (2000). "Structure and mutation analysis of the gene encoding DNA fragmentation factor 40 (caspase-activated nuclease), a candidate neuroblastoma tumour suppressor gene". Hum. Genet. 106 (4): 406–13. doi:10.1007/s004390000257. PMID 10830907.
- Otomo T, Sakahira H, Uegaki K, et al. (2000). "Structure of the heterodimeric complex between CAD domains of CAD and ICAD". Nat. Struct. Biol. 7 (8): 658–62. doi:10.1038/77957. PMID 10932250.
- Durrieu F, Samejima K, Fortune JM, et al. (2001). "DNA topoisomerase IIalpha interacts with CAD nuclease and is involved in chromatin condensation during apoptotic execution". Curr. Biol. 10 (15): 923–6. doi:10.1016/S0960-9822(00)00620-5. PMID 10959840.
- Zhou P, Lugovskoy AA, McCarty JS, et al. (2001). "Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45". Proc. Natl. Acad. Sci. U.S.A. 98 (11): 6051–5. doi:10.1073/pnas.111145098. PMC 33420. PMID 11371636. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=33420.
- Nie Z, Phenix BN, Lum JJ, et al. (2003). "HIV-1 protease processes procaspase 8 to cause mitochondrial release of cytochrome c, caspase cleavage and nuclear fragmentation". Cell Death Differ. 9 (11): 1172–84. doi:10.1038/sj.cdd.4401094. PMID 12404116.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=139241.
- Hsieh SY, Liaw SF, Lee SN, et al. (2003). "Aberrant caspase-activated DNase (CAD) transcripts in human hepatoma cells". Br. J. Cancer 88 (2): 210–6. doi:10.1038/sj.bjc.6600695. PMC 2377037. PMID 12610505. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2377037.
- Liu QL, Kishi H, Ohtsuka K, Muraguchi A (2003). "Heat shock protein 70 binds caspase-activated DNase and enhances its activity in TCR-stimulated T cells". Blood 102 (5): 1788–96. doi:10.1182/blood-2002-11-3499. PMID 12738667.
- Widlak P, Lanuszewska J, Cary RB, Garrard WT (2003). "Subunit structures and stoichiometries of human DNA fragmentation factor proteins before and after induction of apoptosis". J. Biol. Chem. 278 (29): 26915–22. doi:10.1074/jbc.M303807200. PMID 12748178.
- Hillman RT, Green RE, Brenner SE (2005). "An unappreciated role for RNA surveillance". Genome Biol. 5 (2): R8. doi:10.1186/gb-2004-5-2-r8. PMC 395752. PMID 14759258. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=395752.
- Bayascas JR, Yuste VJ, Solé C, et al. (2004). "Characterization of splice variants of human caspase-activated DNase with CIDE-N structure and function". FEBS Lett. 566 (1–3): 234–40. doi:10.1016/j.febslet.2004.04.050. PMID 15147901.
PDB gallery Categories:- Human proteins
- Chromosome 1 gene stubs
Wikimedia Foundation. 2010.